Clinical course of patients with severe COVID-19 pneumonia treated with remdesivir: A real-life study
- PMID: 35482685
- PMCID: PMC9049514
- DOI: 10.1371/journal.pone.0267283
Clinical course of patients with severe COVID-19 pneumonia treated with remdesivir: A real-life study
Abstract
Background: There is currently much uncertainty regarding the most optimal treatment for COVID-19. This study analyze the change in the clinical condition of patients hospitalized for severe COVID-19 pneumonia and treated with remdesivir in a real-life setting, based on the WHO Ordinal Scale. Clinical complications, treatment safety, and impact of other associated drugs were also analyzed.
Methods: We conducted an observational, retrospective study including patients treated with remdesivir. The need for admission to the ICU, the length of ICU and hospital stay, and the need for ventilatory support were analyzed. The laboratory parameters, drugs administered concomitantly, and difference in the length of hospital stay according to the concomitant treatment received were also evaluated. A univariate and multivariate Cox regression analysis was performed to analyze associated factors.
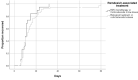
Results: A total of 92 patients were included. The mean length of hospital stay was 15 days, and 90% of the patients had been discharged from the hospital 28 days after starting treatment with remdesivir. The likelihood of hospital discharge among patients not presenting with hypertension as a comorbidity was significantly higher than that of those with this condition (HR = 3.19, P = 0.008). Nineteen patients had to be admitted to the ICU (mean of 18 days). Approximately 11% required invasive mechanical ventilation (mean of 22 days). Almost 37% of the patients received high-flow oxygen therapy and 14% non-invasive mechanical ventilation. Four deaths were recorded within the first week. Main adverse events were increases in transaminase and creatinine levels. Nosocomial infections were more frequent when remdesivir was combined with immunosuppressive drugs.
Conclusions: Patients with severe COVID-19 pneumonia and treated with remdesivir require relatively prolonged hospital stays, many with a need for ventilatory support and, in a considerable proportion of cases, admission to the ICU. However, the observed survival rate is high, and the drug is well tolerated.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Ministerio de Sanidad. Protocolo farmacoclínico del uso de RDV. SNS. 2020 Sep 8. [cited 01 March 2022]. In: AEMPS website [Internet]. Available from: https://www.mscbs.gob.es/profesionales/farmacia/valtermed/docs/20200908_...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources