Remdesivir plus Dexamethasone in COVID-19: A cohort study of severe patients requiring high flow oxygen therapy or non-invasive ventilation
- PMID: 35482703
- PMCID: PMC9049343
- DOI: 10.1371/journal.pone.0267038
Remdesivir plus Dexamethasone in COVID-19: A cohort study of severe patients requiring high flow oxygen therapy or non-invasive ventilation
Abstract
Introduction: Remdesivir and Dexamethasone represent the cornerstone of therapy for critically ill patients with acute hypoxemic respiratory failure caused by Coronavirus Disease 2019 (COVID-19). However, clinical efficacy and safety of concomitant administration of Remdesivir and Dexamethasone (Rem-Dexa) in severe COVID-19 patients on high flow oxygen therapy (HFOT) or non-invasive ventilation (NIV) remains unknown.
Materials and methods: Prospective cohort study that was performed in two medical Intensive Care Units (ICUs) of a tertiary university hospital. The clinical impact of Rem-Dexa administration in hypoxemic patients with COVID-19, who required NIV or HFOT and selected on the simplified acute physiology score II, the sequential organ failure assessment score and the Charlson Comorbidity Index score, was investigated. The primary outcome was 28-day intubation rate; secondary outcomes were end-of-treatment clinical improvement and PaO2/FiO2 ratio, laboratory abnormalities and clinical complications, ICU and hospital length of stay, 28-day and 90-day mortality.
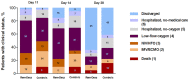
Results: We included 132 patients and found that 28-day intubation rate was significantly lower among Rem-Dexa group (19.7% vs 48.5%, p<0.01). Although the end-of-treatment clinical improvement was larger among Rem-Dexa group (69.7% vs 51.5%, p = 0.05), the 28-day and 90-day mortalities were similar (4.5% and 10.6% vs. 15.2% and 16.7%; p = 0.08 and p = 0.45, respectively). The logistic regression and Cox-regression models showed that concomitant Rem-Dexa therapy was associated with a reduction of 28-day intubation rate (OR 0.22, CI95% 0.05-0.94, p = 0.04), in absence of laboratory abnormalities and clinical complications (p = ns).
Conclusions: In COVID-19 critically ill patients receiving HFO or NIV, 28-day intubation rate was lower in patients who received Rem-Dexa and this finding corresponded to lower end-of-treatment clinical improvement. The individual contribution of either Remdesevir or Dexamethasone to the observed clinical effect should be further investigated.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
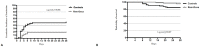
Similar articles
-
Endotracheal intubation rate is lower with additional face-mask noninvasive ventilation for critically-ill SARS-CoV-2 patients requiring high-flow nasal oxygen: a retrospective bicentric cohort with propensity score analysis.Minerva Anestesiol. 2022 Jul-Aug;88(7-8):580-587. doi: 10.23736/S0375-9393.22.16094-3. Epub 2022 Feb 22. Minerva Anestesiol. 2022. PMID: 35191641
-
Impact of dexamethasone on the incidence of ventilator-associated pneumonia in mechanically ventilated COVID-19 patients: a propensity-matched cohort study.Crit Care. 2022 Jun 13;26(1):176. doi: 10.1186/s13054-022-04049-2. Crit Care. 2022. PMID: 35698155 Free PMC article.
-
High-flow nasal oxygen therapy alone or with non-invasive ventilation in immunocompromised patients admitted to ICU for acute hypoxemic respiratory failure: the randomised multicentre controlled FLORALI-IM protocol.BMJ Open. 2019 Aug 10;9(8):e029798. doi: 10.1136/bmjopen-2019-029798. BMJ Open. 2019. PMID: 31401603 Free PMC article.
-
Steroid therapy and antiviral treatment in SARS-CoV-2 pneumonia: clinical contexts and indications.Rev Esp Quimioter. 2022 Apr;35 Suppl 1(Suppl 1):54-58. doi: 10.37201/req/s01.13.2022. Epub 2022 Apr 22. Rev Esp Quimioter. 2022. PMID: 35488828 Free PMC article. Review.
-
Occurrence, Fate, Effects, and Risks of Dexamethasone: Ecological Implications Post-COVID-19.Int J Environ Res Public Health. 2021 Oct 27;18(21):11291. doi: 10.3390/ijerph182111291. Int J Environ Res Public Health. 2021. PMID: 34769808 Free PMC article. Review.
Cited by
-
Early Stage Combination Treatment with Methylprednisolone Pulse and Remdesivir for Severe COVID-19 Pneumonia.Int J Environ Res Public Health. 2023 Jan 7;20(2):1081. doi: 10.3390/ijerph20021081. Int J Environ Res Public Health. 2023. PMID: 36673839 Free PMC article.
-
Identification of potent and orally efficacious phosphodiesterase inhibitors in Cryptosporidium parvum-infected immunocompromised male mice.Nat Commun. 2024 Sep 27;15(1):8272. doi: 10.1038/s41467-024-52658-y. Nat Commun. 2024. PMID: 39333545 Free PMC article.
-
Severe COVID-19 versus multisystem inflammatory syndrome: comparing two critical outcomes of SARS-CoV-2 infection.Eur Respir Rev. 2023 Mar 8;32(167):220197. doi: 10.1183/16000617.0197-2022. Print 2023 Mar 31. Eur Respir Rev. 2023. PMID: 36889788 Free PMC article. Review.
References
-
- Grieco D, Menga L, Cesarano M, Rosà T, Spadaro S, Bitondo M, et al.. Effect of Helmet Noninvasive Ventilation vs High-Flow Nasal Oxygen on Days Free of Respiratory Support in Patients With COVID-19 and Moderate to Severe Hypoxemic Respiratory Failure: The HENIVOT Randomized Clinical Trial. JAMA. 2021;325(17):1731–43. doi: 10.1001/jama.2021.4682 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

