Vibration, a treatment for migraine, linked to calpain driven changes in actin cytoskeleton
- PMID: 35482731
- PMCID: PMC9049534
- DOI: 10.1371/journal.pone.0262058
Vibration, a treatment for migraine, linked to calpain driven changes in actin cytoskeleton
Abstract
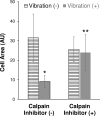
Understanding how a human cell reacts to external physical stimuli is essential to understanding why vibration can elicit localized pain reduction. Stimulation of epithelial cells with external vibration forces has been shown to change cell shape, particularly in regards to structures involved in non-muscle cell motility. We hypothesized that epithelial cells respond to vibration transduction by altering proteins involved in remodeling cytoskeleton. Epithelial cells were exposed to vibration and assessed by microscopy, cytoskeletal staining, immunoblotting and quantitative RT-PCR. Here, we report that epithelial cell lines exposed to 15 minutes of vibration retract filopodia and concentrate actin at the periphery of the cell. In particular, we show an increased expression of the calcium-dependent, cysteine protease, calpain. The discovery that cell transitions are induced by limited exposure to natural forces, such as vibration, provides a foundation to explain how vibrational treatment helps migraine patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Smith KC, Comite SL, Balasubramanian S, Carver A, Liu JF. Vibration anesthesia: a noninvasive method of reducing discomfort prior to dermatologic procedures. Dermatol Online J. 2004. Oct 15;10(2):1. - PubMed
-
- Ahmad M, Mohmand MH. Clinical significance of Vibration Anesthesia on reducing pain of Ring-Block (Subcutaneous Injections) in the patients undergoing Hair Restoration Surgery. Ann Dermatol Res. 2017. Oct 18;1(1):001–5.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

