Durability of mRNA-1273 against COVID-19 in the time of Delta: Interim results from an observational cohort study
- PMID: 35482785
- PMCID: PMC9049574
- DOI: 10.1371/journal.pone.0267824
Durability of mRNA-1273 against COVID-19 in the time of Delta: Interim results from an observational cohort study
Abstract
Background: We conducted a prospective cohort study at Kaiser Permanente Southern California to study the vaccine effectiveness (VE) of mRNA-1273 over time and during the emergence of the Delta variant.
Methods: The cohort for this planned interim analysis consisted of individuals aged ≥18 years receiving 2 doses of mRNA-1273 through June 2021, matched 1:1 to randomly selected unvaccinated individuals by age, sex, and race/ethnicity, with follow-up through September 2021. Outcomes were SARS-CoV-2 infection, and COVID-19 hospitalization and hospital death. Cox proportional hazards models were used to estimate adjusted hazard ratios (aHR) with 95% confidence intervals (CIs) comparing outcomes in the vaccinated and unvaccinated groups. Adjusted VE (%) was calculated as (1-aHR)x100. HRs and VEs were also estimated for SARS-CoV-2 infection by age, sex, race/ethnicity, and during the Delta period (June-September 2021). VE against SARS-CoV-2 infection and COVID-19 hospitalization was estimated at 0-<2, 2-<4, 4-<6, and 6-<8 months post-vaccination.
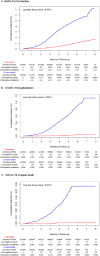
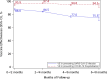
Results: 927,004 recipients of 2 doses of mRNA-1273 were matched to 927,004 unvaccinated individuals. VE (95% CI) was 82.8% (82.2-83.3%) against SARS-CoV-2 infection, 96.1% (95.5-96.6%) against COVID-19 hospitalization, and 97.2% (94.8-98.4%) against COVID-19 hospital death. VE against SARS-CoV-2 infection was similar by age, sex, and race/ethnicity, and was 86.5% (84.8-88.0%) during the Delta period. VE against SARS-CoV-2 infection decreased from 88.0% at 0-<2 months to 75.5% at 6-<8 months.
Conclusions: These interim results provide continued evidence for protection of 2 doses of mRNA-1273 against SARS-CoV-2 infection over 8 months post-vaccination and during the Delta period, and against COVID-19 hospitalization and hospital death.
Conflict of interest statement
AF, LSS, YL, LQ, BKA, GSL, JHK, JET, YT, and HFT are employees of Kaiser Permanente Southern California, which has been contracted by Moderna to conduct this study. KJB is an adjunct investigator at Kaiser Permanente Southern California. CAT is an employee of and a shareholder in Moderna Inc. AF received funding from Pfizer, GlaxoSmithKline, and Gilead unrelated to this manuscript. LSS received funding from GlaxoSmithKline, Dynavax, and Seqirus unrelated to this manuscript. YL received funding from GlaxoSmithKline, Seqirus and Pfizer unrelated to this manuscript. LQ received funding from GlaxoSmithKline and Dynavax unrelated to this manuscript. KJB received funding from GlaxoSmithKline, Dynavax, Pfizer, Gilead, and Seqirus unrelated to this manuscript. BKA received funding from GlaxoSmithKline, Dynavax, Seqirus and Pfizer unrelated to this manuscript. GSL received funding from GlaxoSmithKline unrelated to this manuscript. JHK received funding from GlaxoSmithKline unrelated to this manuscript. JET received funding from Pfizer unrelated to this manuscript. YT received funding from GlaxoSmithKline unrelated to this manuscript. HFT received funding from GlaxoSmithKline and Seqirus unrelated to this manuscript; HFT also served in advisory boards for Janssen and Pfizer. This does not alter our adherence to PLOS ONE policies on sharing data and material.
Figures
References
-
- Bruxvoort KJ, Sy LS, Qian L, Ackerson BK, Luo Y, Lee GS, et al. Real-world effectiveness of the mRNA-1273 vaccine against COVID-19: Interim results from a prospective observational cohort study. Lancet Reg Health Am. 2022;6:100134. Epub 2021/12/02. doi: 10.1016/j.lana.2021.100134 - DOI - PMC - PubMed
-
- Paris C, Perrin S, Hamonic S, Bourget B, Roue C, Brassard O, et al. Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against COVID-19 in healthcare workers: an observational study using surveillance data. Clin Microbiol Infect. 2021;27(11):1699 e5- e8. Epub 2021/07/16. doi: 10.1016/j.cmi.2021.06.043 - DOI - PMC - PubMed
-
- Pilishvili T, Fleming-Dutra KE, Farrar JL, Gierke R, Mohr NM, Talan DA, et al. Interim Estimates of Vaccine Effectiveness of Pfizer-BioNTech and Moderna COVID-19 Vaccines Among Health Care Personnel—33 U.S. Sites, January-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):753–8. Epub 2021/05/21. doi: 10.15585/mmwr.mm7020e2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

