The Giardia ventrolateral flange is a lamellar membrane protrusion that supports attachment
- PMID: 35482847
- PMCID: PMC9089883
- DOI: 10.1371/journal.ppat.1010496
The Giardia ventrolateral flange is a lamellar membrane protrusion that supports attachment
Abstract
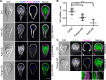
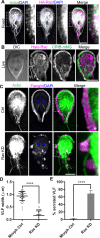
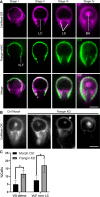
Attachment to the intestinal epithelium is critical to the lifestyle of the ubiquitous parasite Giardia lamblia. The ventrolateral flange is a sheet-like membrane protrusion at the interface between parasites and attached surfaces. This structure has been implicated in attachment, but its role has been poorly defined. Here, we identified a novel actin associated protein with putative WH2-like actin binding domains we named Flangin. Flangin complexes with Giardia actin (GlActin) and is enriched in the ventrolateral flange making it a valuable marker for studying the flanges' role in Giardia biology. Live imaging revealed that the flange grows to around 1 μm in width after cytokinesis, then remains uniform in size during interphase, grows in mitosis, and is resorbed during cytokinesis. A flangin truncation mutant stabilizes the flange and blocks cytokinesis, indicating that flange disassembly is necessary for rapid myosin-independent cytokinesis in Giardia. Rho family GTPases are important regulators of membrane protrusions and GlRac, the sole Rho family GTPase in Giardia, was localized to the flange. Knockdown of Flangin, GlActin, and GlRac result in flange formation defects. This indicates a conserved role for GlRac and GlActin in forming membrane protrusions, despite the absence of canonical actin binding proteins that link Rho GTPase signaling to lamellipodia formation. Flangin-depleted parasites had reduced surface contact and when challenged with fluid shear force in flow chambers they had a reduced ability to remain attached, confirming a role for the flange in attachment. This secondary attachment mechanism complements the microtubule based adhesive ventral disc, a feature that may be particularly important during mitosis when the parental ventral disc disassembles in preparation for cytokinesis. This work supports the emerging view that Giardia's unconventional actin cytoskeleton has an important role in supporting parasite attachment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

