Molecular and neural basis of pleasant touch sensation
- PMID: 35482870
- PMCID: PMC9508691
- DOI: 10.1126/science.abn2479
Molecular and neural basis of pleasant touch sensation
Abstract
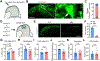
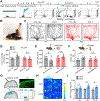
Pleasant touch provides emotional and psychological support that helps mitigate social isolation and stress. However, the underlying mechanisms remain poorly understood. Using a pleasant touch-conditioned place preference (PT-CPP) test, we show that genetic ablation of spinal excitatory interneurons expressing prokineticin receptor 2 (PROKR2), or its ligand PROK2 in sensory neurons, abolishes PT-CPP without impairing pain and itch behaviors in mice. Mutant mice display profound impairments in stress response and prosocial behaviors. Moreover, PROKR2 neurons respond most vigorously to gentle stroking and encode reward value. Collectively, we identify PROK2 as a long-sought neuropeptide that encodes and transmits pleasant touch to spinal PROKR2 neurons. These findings may have important implications for elucidating mechanisms by which pleasant touch deprivation contributes to social avoidance behavior and mental disorders.
Figures




Comment in
-
Sensation of pleasant touch: from molecules to circuits and behaviours.Signal Transduct Target Ther. 2022 Aug 31;7(1):302. doi: 10.1038/s41392-022-01161-1. Signal Transduct Target Ther. 2022. PMID: 36045137 Free PMC article. No abstract available.
References
-
- McGlone F, Wessberg J, Olausson H, Discriminative and affective touch: sensing and feeling. Neuron 82, 737–755 (2014). - PubMed
-
- Morrison I, Loken LS, Olausson H, The skin as a social organ. Experimental Brain Research 204, 305–314 (2010). - PubMed
-
- Montagu A, Touching: The Human Significance of the Skin. (HarperCollins; New York, ed. 3rd ed, 1986).
-
- Harlow HF, Suomi SJ, Nature of love--simplified. Am Psychol 25, 161–168 (1970). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

