Preinfusion factors impacting relapse immunophenotype following CD19 CAR T cells
- PMID: 35482927
- PMCID: PMC9979750
- DOI: 10.1182/bloodadvances.2022007423
Preinfusion factors impacting relapse immunophenotype following CD19 CAR T cells
Abstract
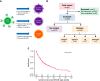
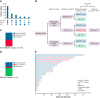
Relapse following chimeric antigen receptor (CAR) T-cell therapy directed against CD19 for relapsed/refractory B-acute lymphoblastic leukemia (r/r B-ALL) remains a significant challenge. Three main patterns of relapse predominate: CD19 positive (CD19pos) relapse, CD19 negative (CD19neg) relapse, and lineage switch (LS). Development and validation of risk factors that predict relapse phenotype could help define potential pre- or post-CAR T-cell infusion interventions aimed at decreasing relapse. Our group sought to extensively characterize preinfusion risk factors associated with the development of each relapse pattern via a multicenter, retrospective review of children and young adults with r/r B-ALL treated with a murine-based CD19-CAR construct. Of 420 patients treated with CAR, 166 (39.5%) relapsed, including 83 (50%) CD19pos, 68 (41%) CD19neg, and 12 (7.2%) LS relapses. A greater cumulative number of prior complete remissions was associated with CD19pos relapses, whereas high preinfusion disease burden, prior blinatumomab nonresponse, older age, and 4-1BB CAR construct were associated with CD19neg relapses. The presence of a KMT2A rearrangement was the only preinfusion risk factor associated with LS. The median overall survival following a post-CAR relapse was 11.9 months (95% CI, 9-17) and was particularly dismal in patients experiencing an LS, with no long-term survivors following this pattern of relapse. Given the poor outcomes for those with post-CAR relapse, study of relapse prevention strategies, such as consolidative hematopoietic stem cell transplantation, is critical and warrants further investigation on prospective clinical trials.
Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.J.B. received honoraria from Amgen and Blueprint Medicines. D.W.L. has consulted for Harpoon Therapeutics, advised for Amgen and BMS, and received research funding from Kite Pharma and Gilead. S.A.G. received research funding from Novartis, Kite, Vertex, and Servier, has consulted for Novartis, Roche, GSK, Humanigen, CBMG, Eureka, Janssen/JNJ, and Jazz Pharmaceuticals, and has advised for Novartis, Adaptimmune, TCR2, Cellectis, Juno, Vertex, Allogene, Jazz pharmaceuticals, and Cabaletta. M.R.V. advised for Novartis, Equillium, Mederus, and Takeda. L.G. has consulted for Amgen, Novartis, and Roche and advised for Amgen, Novartis, and Celgene and holds equity in Amgen, Anchiano, Blueprint Medicines, Celgene, Clovis, Mirati, and Sanofi Paris. P.A.B has advised for Novartis, Takeda, Amegen, Kura, and Kite. S.R.R. received research funding from Pfizer. M.A.P has advised for Mesoblast, Novartis, Equillium, Medexus, and Vertex, received research funding from Adaptive and Miltenyi, and received honoraria from Novartis, Miltenyi, and Bellicum. R.A.G. has consulted for Novartis and received patents and royalties from BMS. T.W.L. has advised for Bayer, Cellectis, Novartis, Deciphera, Juno, and Y-mAbs Therapeutics, received honoraria from Bayer, Cellectis, Novartis, Deciphera, Juno, and Y-mAbs Therapeutics, and received research support from Pfizer and Bayer.
The current affiliation for A.T. is Janssen Research & Development, LLC, Raritan, NJ.
Figures



Similar articles
-
Factors Impacting Overall and Event-Free Survival following Post-Chimeric Antigen Receptor T Cell Consolidative Hematopoietic Stem Cell Transplantation.Transplant Cell Ther. 2022 Jan;28(1):31.e1-31.e9. doi: 10.1016/j.jtct.2021.10.011. Epub 2021 Oct 20. Transplant Cell Ther. 2022. PMID: 34687939 Free PMC article.
-
Blinatumomab Nonresponse and High-Disease Burden Are Associated With Inferior Outcomes After CD19-CAR for B-ALL.J Clin Oncol. 2022 Mar 20;40(9):932-944. doi: 10.1200/JCO.21.01405. Epub 2021 Nov 12. J Clin Oncol. 2022. PMID: 34767461 Free PMC article.
-
Impact of Consolidative Unrelated Cord Blood Transplantation on Clinical Outcomes of Patients With Relapsed/Refractory Acute B Lymphoblastic Leukemia Entering Remission Following CD19 Chimeric Antigen Receptor T Cells.Front Immunol. 2022 Apr 26;13:879030. doi: 10.3389/fimmu.2022.879030. eCollection 2022. Front Immunol. 2022. PMID: 35558072 Free PMC article.
-
Chimeric Antigen Receptor T Cell Therapy for Pediatric B-ALL: Narrowing the Gap Between Early and Long-Term Outcomes.Front Immunol. 2020 Aug 11;11:1985. doi: 10.3389/fimmu.2020.01985. eCollection 2020. Front Immunol. 2020. PMID: 32849662 Free PMC article. Review.
-
Role of chimeric antigen receptor T-cell therapy: bridge to transplantation or stand-alone therapy in pediatric acute lymphoblastic leukemia.Curr Opin Hematol. 2021 Nov 1;28(6):373-379. doi: 10.1097/MOH.0000000000000685. Curr Opin Hematol. 2021. PMID: 34508031 Free PMC article. Review.
Cited by
-
Advancing Diagnostics and Therapy to Reach Universal Cure in Childhood ALL.J Clin Oncol. 2023 Dec 20;41(36):5579-5591. doi: 10.1200/JCO.23.01286. Epub 2023 Oct 11. J Clin Oncol. 2023. PMID: 37820294 Free PMC article. Review.
-
Acute lymphoblastic leukaemia.Nat Rev Dis Primers. 2024 Jun 13;10(1):41. doi: 10.1038/s41572-024-00525-x. Nat Rev Dis Primers. 2024. PMID: 38871740 Review.
-
Potency assays and biomarkers for cell-based advanced therapy medicinal products.Front Immunol. 2023 Jun 9;14:1186224. doi: 10.3389/fimmu.2023.1186224. eCollection 2023. Front Immunol. 2023. PMID: 37359560 Free PMC article. Review.
-
A Review of CAR-T Therapy in Pediatric and Young Adult B-Lineage Acute Leukemia: Clinical Perspectives in Singapore.Onco Targets Ther. 2023 Mar 14;16:165-176. doi: 10.2147/OTT.S271373. eCollection 2023. Onco Targets Ther. 2023. PMID: 36941828 Free PMC article. Review.
-
Failure of ALL recognition by CAR T cells: a review of CD 19-negative relapses after anti-CD 19 CAR-T treatment in B-ALL.Front Immunol. 2023 Apr 14;14:1165870. doi: 10.3389/fimmu.2023.1165870. eCollection 2023. Front Immunol. 2023. PMID: 37122700 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

