Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy
- PMID: 35483010
- PMCID: PMC9362876
- DOI: 10.1200/JCO.22.00032
Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy
Abstract
Purpose: Prospective data on the efficacy of a watch-and-wait strategy to achieve organ preservation in patients with locally advanced rectal cancer treated with total neoadjuvant therapy are limited.
Methods: In this prospective, randomized phase II trial, we assessed the outcomes of 324 patients with stage II or III rectal adenocarcinoma treated with induction chemotherapy followed by chemoradiotherapy (INCT-CRT) or chemoradiotherapy followed by consolidation chemotherapy (CRT-CNCT) and either total mesorectal excision (TME) or watch-and-wait on the basis of tumor response. Patients in both groups received 4 months of infusional fluorouracil-leucovorin-oxaliplatin or capecitabine-oxaliplatin and 5,000 to 5,600 cGy of radiation combined with either continuous infusion fluorouracil or capecitabine during radiotherapy. The trial was designed as two stand-alone studies with disease-free survival (DFS) as the primary end point for both groups, with a comparison to a null hypothesis on the basis of historical data. The secondary end point was TME-free survival.
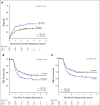
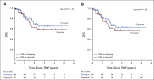
Results: Median follow-up was 3 years. Three-year DFS was 76% (95% CI, 69 to 84) for the INCT-CRT group and 76% (95% CI, 69 to 83) for the CRT-CNCT group, in line with the 3-year DFS rate (75%) observed historically. Three-year TME-free survival was 41% (95% CI, 33 to 50) in the INCT-CRT group and 53% (95% CI, 45 to 62) in the CRT-CNCT group. No differences were found between groups in local recurrence-free survival, distant metastasis-free survival, or overall survival. Patients who underwent TME after restaging and patients who underwent TME after regrowth had similar DFS rates.
Conclusion: Organ preservation is achievable in half of the patients with rectal cancer treated with total neoadjuvant therapy, without an apparent detriment in survival, compared with historical controls treated with chemoradiotherapy, TME, and postoperative chemotherapy.
Trial registration: ClinicalTrials.gov NCT02008656.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures



Comment in
-
Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: Induction or Consolidation Chemotherapy?J Clin Oncol. 2022 Aug 10;40(23):2515-2519. doi: 10.1200/JCO.22.00506. Epub 2022 Jun 20. J Clin Oncol. 2022. PMID: 35724356 No abstract available.
-
Interpretation of the OPRA Study: Are Our Criteria Reliable Enough to Recommend Watch and Wait?J Clin Oncol. 2023 Jan 10;41(2):416-417. doi: 10.1200/JCO.22.01260. Epub 2022 Sep 20. J Clin Oncol. 2023. PMID: 36126234 No abstract available.
-
Total Neoadjuvant Therapy for All Rectal Cancers: Is This the Way Ahead of the OPRA Trial?J Clin Oncol. 2023 Jan 10;41(2):415-416. doi: 10.1200/JCO.22.01166. Epub 2022 Sep 20. J Clin Oncol. 2023. PMID: 36126238 No abstract available.
-
Radio- or Chemotherapy Dose Escalation for Organ Preservation in Rectal Cancer?J Clin Oncol. 2023 Jan 10;41(2):417. doi: 10.1200/JCO.22.01296. Epub 2022 Sep 20. J Clin Oncol. 2023. PMID: 36126242 No abstract available.
-
Hits and Misses in Novel Pancreatic and Rectal Cancer Treatment Options.Int J Radiat Oncol Biol Phys. 2023 Mar 1;115(3):545-552. doi: 10.1016/j.ijrobp.2022.10.022. Int J Radiat Oncol Biol Phys. 2023. PMID: 36725162 No abstract available.
References
-
- Benson AB, Venook AP, Al-Hawary MM, et al. : NCCN guidelines insights: Rectal cancer, version 6.2020. J Natl Compr Canc Netw 18:806-815, 2020 - PubMed
-
- Peeters KC, van de Velde CJ, Leer JW, et al. : Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients—A Dutch colorectal cancer group study. J Clin Oncol 23:6199-6206, 2005 - PubMed
-
- Maas M, Nelemans PJ, Valentini V, et al. : Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol 11:835-844, 2010 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

