Traceless Benzylic C-H Amination via Bifunctional N-Aminopyridinium Intermediates
- PMID: 35483017
- PMCID: PMC9256810
- DOI: 10.1002/anie.202200665
Traceless Benzylic C-H Amination via Bifunctional N-Aminopyridinium Intermediates
Abstract
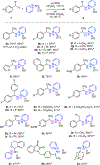
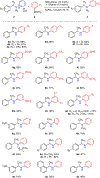
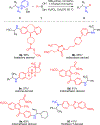
C-H amination reactions provide the opportunity to streamline the synthesis of nitrogen-containing organic small molecules. The impact of intermolecular C-H amination methods, however, is currently limited the frequent requirement for the amine precursors to bear activating groups, such as N-sulfonyl substituents, that are both challenging to remove and not useful synthetic handles for subsequent derivatization. Here, we introduce traceless nitrogen activation for C-H amination-which enables application of selective C-H amination chemistry to the preparation of diverse N-functionalized products-via sequential benzylic C-H N-aminopyridylation followed by Ni-catalyzed C-N cross-coupling with aryl boronic acids. Unlike many C-H amination reactions that provide access to protected amines, the current method installs an easily diversifiable synthetic handle that serves as a lynchpin for C-H amination, deaminative N-N functionalization sequences.
Keywords: Amination; Cross-Coupling; Nickel; Nitrene Transfer; Synthetic Methods.
© 2022 Wiley-VCH GmbH.
Figures


References
-
- Jiao J, Murakami K, Itami K, ACS Catal. 2016, 6, 610–633;
- Singh R, Mukherjee A, ACS Catal. 2019, 9, 3604–3617;
- Park Y, Kim Y, Chang S, Chem. Rev 2017, 117, 9247–9301; - PubMed
- Collet F, Dodd RH, Dauban P, Chem. Commun 2009, 5061–5074; - PubMed
- Louillat M-L, Patureau FW, Chem. Soc. Rev 2014, 43, 901–910; - PubMed
- Du Bois J, Org. Process. Res. Dev 2011, 15, 758–762. - PMC - PubMed
-
-
For selected examples, see:
Jia Z-J, Gao S, Arnold FH, J. Am. Chem. Soc 2020, 142, 10279–10283;
Clark JR, Feng K, Sookezian A, White MC, Nat. Chem 2018, 10, 583–591;
Ide T, Feng K, Dixon CF, Teng D, Clark JR, Han W, Wendell CI, Koch V, White MC, J. Am. Chem. Soc 2021, 143, 14969–14975;
Williams KW, Du Bois J, J. Am. Chem. Soc 2007, 129, 562–568;
Bess EN, DeLuca RJ, Tindall DJ, Oderinde MS, Roizen JL, Du Bois J, Sigman MS, J. Am. Chem. Soc 2014, 136, 5783–5789;
Liang C, Robert-Peillard F, Fruit C, Müller P, Dodd RH, Dauben P, Angew. Chem 2006, 118, 4757–4760; Angew. Chem. Int. Ed. 2006, 45, 4641–4644;
Chen S-J, Golden DL, Krska SW, Stahl SS, J. Am. Chem. Soc 2021, 143, 14438–14444.
-
-
-
Similar N-activation is often not required in intramolecular C–H amination reactions, see:
Jeffrey JL, Sarpong R, Chem. Sci 2013, 4, 4092–4106.
-
-
-
Recent progress has been made towards the installation of unsubstituted NH2 groups via C–H amination, see:
Paudyal MP, Adebesin AM, Burt SR, Ess DH, Ma Z, Kürti L, Falck JR, Science 2016, 353, 1144–1147;
See YY, Sanford MS, Org. Lett 2020, 22, 2931–2934.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

