Factors that influence singlet oxygen formation vs. ligand substitution for light-activated ruthenium anticancer compounds
- PMID: 35483128
- PMCID: PMC9133143
- DOI: 10.1016/j.cbpa.2022.102143
Factors that influence singlet oxygen formation vs. ligand substitution for light-activated ruthenium anticancer compounds
Abstract
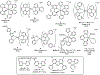
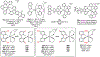
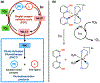
This review focuses on light-activated ruthenium anticancer compounds and the factors that influence which pathway is favored. Photodynamic therapy (PDT) is favored by π expansion and the presence of low-lying triplet excited states (e.g. 3MLCT, 3IL). Photoactivated chemotherapy (PACT) refers to light-driven ligand dissociation to give a toxic metal complex or a toxic ligand upon photo substitution. This process is driven by steric bulk near the metal center and weak metal-ligand bonds to create a low-energy 3MC state with antibonding character. With protic dihydroxybipyridine ligands, ligand charge can play a key role in these processes, with a more electron-rich deprotonated ligand favoring PDT and an electron-poor protonated ligand favoring PACT in several cases.
Keywords: Anticancer; Diimine ligands; Photoactivated chemotherapy; Photochemistry; Photodissociation; Photodynamic therapy; Photosubstitution; Protic ligands; Ruthenium; pH responsive.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Gaiddon C, Pfeffer M, The Fate of Cycloruthenated Compounds: From C-H Activation to Innovative Anticancer Therapy, Eur. J. Inorg. Chem, 2017 (2017) 1–17.
-
- Hartinger CG, Zorbas-Seifried S, Jakupec MA, Kynast B, Zorbas H, Keppler BK, From bench to bedside – preclinical and early clinical development of the anticancer agent indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)] (KP1019 or FFC14A), J. Inorg. Biochem, 100 (2006) 891–904. - PubMed
-
- Trondl R, Heffeter P, Kowol CR, Jakupec MA, Berger W, Keppler BK, NKP-1339, the first ruthenium-based anticancer drug on the edge to clinical application, Chem. Sci, 5 (2014) 2925–2932.
-
- Bergamo A, Sava G, Ruthenium anticancer compounds: myths and realities of the emerging metal-based drugs, Dalton Trans., 40 (2011) 7817. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

