Pregnancy and COVID-19, focus on vaccine and pharmacological treatment
- PMID: 35483212
- PMCID: PMC9023094
- DOI: 10.1016/j.jri.2022.103630
Pregnancy and COVID-19, focus on vaccine and pharmacological treatment
Abstract
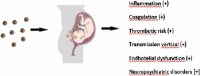
The global pandemic of COVID-19 is currently ongoing. Clinical evidence shows that specific population groups such as the elderly, individuals with comorbidities, and pregnant women may be at increased risk for infection and serious complications. In particular, physiologic changes during pregnancy may be significant on the immune and respiratory systems and progression of COVID-19 disease. Pregnant women are routinely excluded from pre-registration clinical trials, this potentially limits their access to therapies through off-label or compassionate use. Vaccination remains an important pillar of the response to COVID-19, particularly as variants of the virus continue to spread across countries. Growing evidence indicates that COVID-19 mRNA vaccines do not cause pregnancy complications for expectant mothers and their infants. In this brief review, we explore current knowledge about COVID-19 in pregnancy by highlighting current recommendations for vaccination and drug treatments.
Keywords: COVID-19; Drugs; Pregnancy; Vaccine.
Copyright © 2022 Elsevier B.V. All rights reserved.
Figures
Comment in
-
Comment to "Pregnancy and COVID-19, focus on vaccine and pharmacological treatment".J Reprod Immunol. 2022 Aug;152:103639. doi: 10.1016/j.jri.2022.103639. Epub 2022 May 10. J Reprod Immunol. 2022. PMID: 35569346 Free PMC article. No abstract available.
Similar articles
-
Anti-SARS-CoV-2 vaccination strategy for pregnant women in Japan.J Obstet Gynaecol Res. 2021 Jun;47(6):1958-1964. doi: 10.1111/jog.14748. Epub 2021 Mar 23. J Obstet Gynaecol Res. 2021. PMID: 33754418 Free PMC article.
-
[Technical guidelines for seasonal influenza vaccination in China (2021-2022)].Zhonghua Liu Xing Bing Xue Za Zhi. 2021 Oct 10;42(10):1722-1749. doi: 10.3760/cma.j.cn112338-20210913-00732. Zhonghua Liu Xing Bing Xue Za Zhi. 2021. PMID: 34814607 Chinese.
-
[Technical guidelines for seasonal influenza vaccination in China (2022-2023)].Zhonghua Yu Fang Yi Xue Za Zhi. 2022 Oct 6;56(10):1356-1386. doi: 10.3760/cma.j.cn112150-20220825-00840. Zhonghua Yu Fang Yi Xue Za Zhi. 2022. PMID: 36274602 Chinese.
-
The impact of COVID-19 in pregnancy: Part II. Vaccination to pregnant women.J Chin Med Assoc. 2021 Oct 1;84(10):903-910. doi: 10.1097/JCMA.0000000000000612. J Chin Med Assoc. 2021. PMID: 34433191 Review.
-
[Vaccination against influenza in pregnant women - safety and effectiveness].Ginekol Pol. 2013 Jan;84(1):56-61. doi: 10.17772/gp/1541. Ginekol Pol. 2013. PMID: 23488311 Review. Polish.
Cited by
-
The Community Pharmacy as a Study Center for the Epidemiological Analysis of the Population Vaccination against SARS-CoV-2: Evaluation of Vaccine Safety and Pharmaceutical Service.Pharmacy (Basel). 2024 Jan 20;12(1):16. doi: 10.3390/pharmacy12010016. Pharmacy (Basel). 2024. PMID: 38392923 Free PMC article.
-
An Update on COVID-19 Vaccination and Pregnancy.J Pers Med. 2023 May 6;13(5):797. doi: 10.3390/jpm13050797. J Pers Med. 2023. PMID: 37240967 Free PMC article. Review.
-
COVID-19 Vaccination Acceptance During Pregnancy in Europe.Cureus. 2024 Jul 1;16(7):e63562. doi: 10.7759/cureus.63562. eCollection 2024 Jul. Cureus. 2024. PMID: 39087190 Free PMC article. Review.
-
COVID-19 and Pregnancy.Discoveries (Craiova). 2022 Jun 30;10(2):e147. doi: 10.15190/d.2022.6. eCollection 2022 Apr-Jun. Discoveries (Craiova). 2022. PMID: 36438440 Free PMC article. Review.
-
Pharmacological Agents with Antiviral Activity against Monkeypox Infection.Int J Mol Sci. 2022 Dec 14;23(24):15941. doi: 10.3390/ijms232415941. Int J Mol Sci. 2022. PMID: 36555584 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical