GPCR-mediated β-arrestin activation deconvoluted with single-molecule precision
- PMID: 35483373
- PMCID: PMC9191627
- DOI: 10.1016/j.cell.2022.03.042
GPCR-mediated β-arrestin activation deconvoluted with single-molecule precision
Abstract
β-arrestins bind G protein-coupled receptors to terminate G protein signaling and to facilitate other downstream signaling pathways. Using single-molecule fluorescence resonance energy transfer imaging, we show that β-arrestin is strongly autoinhibited in its basal state. Its engagement with a phosphopeptide mimicking phosphorylated receptor tail efficiently releases the β-arrestin tail from its N domain to assume distinct conformations. Unexpectedly, we find that β-arrestin binding to phosphorylated receptor, with a phosphorylation barcode identical to the isolated phosphopeptide, is highly inefficient and that agonist-promoted receptor activation is required for β-arrestin activation, consistent with the release of a sequestered receptor C tail. These findings, together with focused cellular investigations, reveal that agonism and receptor C-tail release are specific determinants of the rate and efficiency of β-arrestin activation by phosphorylated receptor. We infer that receptor phosphorylation patterns, in combination with receptor agonism, synergistically establish the strength and specificity with which diverse, downstream β-arrestin-mediated events are directed.
Keywords: G protein-coupled receptor; GPCR; agonist; arrestin; conformational dynamics; phosphorylation; phosphorylation barcode; receptor signaling; single-molecule FRET; β-arrestin.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.C.B. has an equity interest in Lumidyne Technologies.
Figures
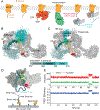

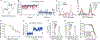


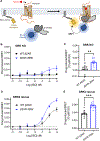
Comment in
-
A twist in β-arrestin's tail.Sci Signal. 2022 May 24;15(735):eadd1034. doi: 10.1126/scisignal.add1034. Epub 2022 May 24. Sci Signal. 2022. PMID: 35609126
-
Dynamic mechanism of GPCR-mediated β-arrestin: a potential therapeutic agent discovery of biased drug.Signal Transduct Target Ther. 2022 Aug 12;7(1):283. doi: 10.1038/s41392-022-01140-6. Signal Transduct Target Ther. 2022. PMID: 35961979 Free PMC article. No abstract available.
References
-
- Attramadal H, Arriza JL, Aoki C, Dawson TM, Codina J, Kwatra MM, Snyder SH, Caron MG, and Lefkowitz RJ (1992). Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J. Biol. Chem 267, 17882–17890. - PubMed
-
- Blanchard SC, Gonzalez RL, Kim HD, Chu S, and Puglisi JD (2004). tRNA selection and kinetic proofreading in translation. Nat. Struct. Mol. Biol 11, 1008–1014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

