Enhanced safety and efficacy of protease-regulated CAR-T cell receptors
- PMID: 35483375
- PMCID: PMC9467936
- DOI: 10.1016/j.cell.2022.03.041
Enhanced safety and efficacy of protease-regulated CAR-T cell receptors
Abstract
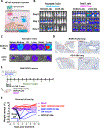
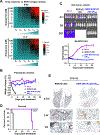
Regulatable CAR platforms could circumvent toxicities associated with CAR-T therapy, but existing systems have shortcomings including leakiness and attenuated activity. Here, we present SNIP CARs, a protease-based platform for regulating CAR activity using an FDA-approved small molecule. Design iterations yielded CAR-T cells that manifest full functional capacity with drug and no leaky activity in the absence of drug. In numerous models, SNIP CAR-T cells were more potent than constitutive CAR-T cells and showed diminished T cell exhaustion and greater stemness. In a ROR1-based CAR lethality model, drug cessation following toxicity onset reversed toxicity, thereby credentialing the platform as a safety switch. In the same model, reduced drug dosing opened a therapeutic window that resulted in tumor eradication in the absence of toxicity. SNIP CARs enable remote tuning of CAR activity, which provides solutions to safety and efficacy barriers that are currently limiting progress in using CAR-T cells to treat solid tumors.
Keywords: CAR-T therapy; T cell differentiation; T cell exhaustion; brain tumors; cellular immunotherapy; chimeric antigen receptors; immune cell engineering; solid cancers; synthetic biology.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.L., R.G.M., M.Z.L., and C.L.M. are coinventors on a patent related to this work. C.L.M. is a cofounder of Lyell Immunopharma, Syncopation Life Sciences, and Link Cell Therapies, which are developing CAR-based therapies, and consults for Lyell, NeoImmune Tech, Apricity, Nektar, Immatics, Ensoma, Mammoth, Glaxo Smith Kline, and Bristol Myers Squibb. L.L., R.G.M., E.S., and E.W.W. are consultants for and hold equity in Lyell Immunopharma. L.L. is a cofounder of, consults for, and holds equity in Syncopation Life Sciences. R.G.M. is a cofounder of, consults for, and holds equity in Syncopation Life Sciences and Link Cell Therapies. R.G.M. is a consultant for Illumina Radiopharmaceuticals, NKarta, ImmunAI, Arovella Therapeutics, Zai Lab, and Aptorum Group. R.G.M. serves on the Data and Safety Monitoring Board for Fate Therapeutics. J.T. is a consultant for Dorian Therapeutics. E.W.W. consults for and holds equity in VISTAN Health. A.T.S. is a founder of Immunai and Cartography Biosciences and receives research funding from Arsenal Biosciences, Allogene Therapeutics, and 10x Genomics. K.R.P. is a cofounder and employee of Cartography Biosciences. H.Y.C. is a cofounder of Accent Therapeutics, Boundless Bio, and Cartography Biosciences and is an advisor to 10x Genomics, Arsenal Biosciences, and Spring Discovery. J.R.C. is a cofounder and equity holder of Trapeze Therapeutics, Combangio, and Virsti Therapeutics; he has financial interests in Aravive, Xyence Therapeutics, and Syncopation Life Sciences; and he is a member of the Board of Directors of Ligand Pharmaceuticals and Revel Pharmaceuticals. S.A.Y.-H. is a consultant for Trapeze Therapeutics and Xyence Therapeutics.
Figures





Comment in
-
A SNIPpet of safety: a Goldilocks approach in CAR-T therapy.Cell Res. 2022 Jul;32(7):603-604. doi: 10.1038/s41422-022-00682-2. Cell Res. 2022. PMID: 35739237 Free PMC article. No abstract available.
Similar articles
-
Adoptive cell therapy for solid tumors beyond CAR-T: Current challenges and emerging therapeutic advances.J Control Release. 2024 Apr;368:372-396. doi: 10.1016/j.jconrel.2024.02.033. Epub 2024 Mar 6. J Control Release. 2024. PMID: 38408567 Review.
-
High-performance multiplex drug-gated CAR circuits.Cancer Cell. 2022 Nov 14;40(11):1294-1305.e4. doi: 10.1016/j.ccell.2022.08.008. Epub 2022 Sep 8. Cancer Cell. 2022. PMID: 36084652 Free PMC article.
-
Regional Delivery of Chimeric Antigen Receptor-Engineered T Cells Effectively Targets HER2+ Breast Cancer Metastasis to the Brain.Clin Cancer Res. 2018 Jan 1;24(1):95-105. doi: 10.1158/1078-0432.CCR-17-2041. Epub 2017 Oct 23. Clin Cancer Res. 2018. PMID: 29061641 Free PMC article.
-
Tuning CARs: recent advances in modulating chimeric antigen receptor (CAR) T cell activity for improved safety, efficacy, and flexibility.J Transl Med. 2023 Mar 15;21(1):197. doi: 10.1186/s12967-023-04041-6. J Transl Med. 2023. PMID: 36922828 Free PMC article. Review.
-
Engineering CAR-T Cells for Next-Generation Cancer Therapy.Cancer Cell. 2020 Oct 12;38(4):473-488. doi: 10.1016/j.ccell.2020.07.005. Epub 2020 Jul 30. Cancer Cell. 2020. PMID: 32735779 Review.
Cited by
-
DeltaRex-G, tumor targeted retrovector encoding a CCNG1 inhibitor, for CAR-T cell therapy induced cytokine release syndrome.Front Mol Med. 2024 Sep 18;4:1461151. doi: 10.3389/fmmed.2024.1461151. eCollection 2024. Front Mol Med. 2024. PMID: 39359418 Free PMC article.
-
CAR‑T cell therapy: A breakthrough in traditional cancer treatment strategies (Review).Mol Med Rep. 2024 Mar;29(3):47. doi: 10.3892/mmr.2024.13171. Epub 2024 Jan 26. Mol Med Rep. 2024. PMID: 38275119 Free PMC article. Review.
-
Editor's pick: Cargo Therapeutics.Nat Biotechnol. 2024 Sep;42(9):1339-1340. doi: 10.1038/s41587-024-02358-1. Nat Biotechnol. 2024. PMID: 39160321 No abstract available.
-
Probiotic-guided CAR-T cells for solid tumor targeting.Science. 2023 Oct 13;382(6667):211-218. doi: 10.1126/science.add7034. Epub 2023 Oct 12. Science. 2023. PMID: 37824640 Free PMC article.
-
Engineering enhanced chimeric antigen receptor-T cell therapy for solid tumors.Immunooncol Technol. 2023 May 24;19:100385. doi: 10.1016/j.iotech.2023.100385. eCollection 2023 Sep. Immunooncol Technol. 2023. PMID: 37483659 Free PMC article. Review.
References
-
- Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, and Tamada K (2018). IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. - PubMed
-
- Benjamin R, Graham C, Yallop D, Jozwik A, Mirci-Danicar OC, Lucchini G, Pinner D, Jain N, Kantarjian H, Boissel N, et al. (2020). Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet 396, 1885–1894. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

