Early neurodevelopment of HIV-exposed uninfected children in the era of antiretroviral therapy: a systematic review and meta-analysis
- PMID: 35483380
- PMCID: PMC9090907
- DOI: 10.1016/S2352-4642(22)00071-2
Early neurodevelopment of HIV-exposed uninfected children in the era of antiretroviral therapy: a systematic review and meta-analysis
Abstract
Background: There are 15·4 million children who are HIV-exposed and uninfected worldwide. Early child development crucially influences later academic and socioeconomic factors. However, the neurodevelopmental outcomes of HIV-exposed uninfected (HEU) children in the era of maternal antiretroviral therapy (ART) remain unclear. We aimed to examine the effects of in-utero exposure to HIV and ART on child neurodevelopment.
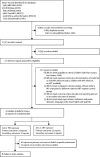
Methods: For this systematic review and meta-analysis, we searched MEDLINE, Embase, PubMed, Africa-Wide Information, PsycInfo, and Global Health databases from inception to May 27, 2020, for studies from the past two decades reporting neurodevelopment of HEU children aged 0-5 years compared with HIV-unexposed (HU) children (aim 1), and effects of different maternal ART regimens on neurodevelopment of HEU children (aim 2). We did narrative syntheses for both aims, and a random-effects meta-analysis of high-quality studies comparing HEU children and HU children, to obtain weighted pooled estimates of effect sizes. This study was registered with PROSPERO, CRD42018075910.
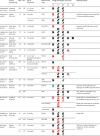
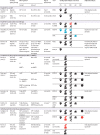
Findings: We screened 35 527 records and included 45 articles from 31 studies. Overall, 12 (57%) of 21 studies comparing HEU children and HU children found worse neurodevelopment in HEU children in at least one domain. Study design and methodological quality were variable, with heterogeneity across populations. Meta-analysis included eight high-quality studies comparing 1856 HEU children with 3067 HU children at ages 12-24 months; among HEU children with available data, 1709 (99%) of 1732 were exposed to ART. HEU children had poorer expressive language (effect size -0·17 [95% CI -0·27 to -0·07], p=0·0013) and gross motor function (-0·13 [-0·20 to -0·07], p<0·0001) than HU children, but similar cognitive development (-0·06 [-0·19 to 0·06], p=0·34), receptive language development (-0·10 [-0·23 to 0·03], p=0·14), and fine motor skills (-0·05 [-0·15 to 0·06], p=0·36). Results suggested little or no evidence of an effect of specific maternal ART regimens on neurodevelopment; study heterogeneity prevented meta-analysis.
Interpretation: HEU children are at risk of subtle impairments in expressive language and gross motor development by age 2 years. We found no consistent effect of maternal ART regimens analysed, although evidence was scarce. We highlight the need for large high-quality longitudinal studies to assess the neurodevelopmental trajectories of HEU children and to investigate underlying mechanisms to inform intervention strategies.
Funding: Wellcome Trust and Medical Research Council.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AJP declares paid participation on the Botnar Research Centre for Child Health independent external review board and is a member of several data and safety monitoring boards with no payment, none of which relate to the current research. DJS has received research grants or consultancy honoraria from Discovery, Johnson & Johnson, Lundbeck, Sanofi, Servier, Takeda, and Vistagen. All other authors declare no competing interests.
Figures



References
-
- Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect Dis. 2016;16:e92–107. - PubMed
-
- Mofenson LM. Editorial commentary: new challenges in the elimination of pediatric HIV infection: the expanding population of HIV-exposed but uninfected children. Clin Infect Dis. 2015;60:1357–1360. - PubMed
-
- Le Doaré K, Bland R, Newell ML. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics. 2012;130:e1326–e1344. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

