Inflammasome activation in infected macrophages drives COVID-19 pathology
- PMID: 35483404
- PMCID: PMC9288243
- DOI: 10.1038/s41586-022-04802-1
Inflammasome activation in infected macrophages drives COVID-19 pathology
Abstract
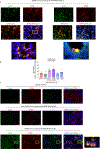
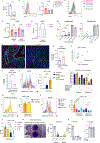
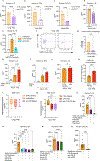
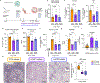
Severe COVID-19 is characterized by persistent lung inflammation, inflammatory cytokine production, viral RNA and a sustained interferon (IFN) response, all of which are recapitulated and required for pathology in the SARS-CoV-2-infected MISTRG6-hACE2 humanized mouse model of COVID-19, which has a human immune system1-20. Blocking either viral replication with remdesivir21-23 or the downstream IFN-stimulated cascade with anti-IFNAR2 antibodies in vivo in the chronic stages of disease attenuates the overactive immune inflammatory response, especially inflammatory macrophages. Here we show that SARS-CoV-2 infection and replication in lung-resident human macrophages is a critical driver of disease. In response to infection mediated by CD16 and ACE2 receptors, human macrophages activate inflammasomes, release interleukin 1 (IL-1) and IL-18, and undergo pyroptosis, thereby contributing to the hyperinflammatory state of the lungs. Inflammasome activation and the accompanying inflammatory response are necessary for lung inflammation, as inhibition of the NLRP3 inflammasome pathway reverses chronic lung pathology. Notably, this blockade of inflammasome activation leads to the release of infectious virus by the infected macrophages. Thus, inflammasomes oppose host infection by SARS-CoV-2 through the production of inflammatory cytokines and suicide by pyroptosis to prevent a productive viral cycle.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing financial interests
RAF is an advisor to Glaxo Smith Kline, Zai Labs, and Ventus Therapeutics. JL is an advisor of Ventus Therapeutics. SH is a consultant for FORMA Therapeutics. All other authors declare no competing financial interests.
Figures















Update of
-
Inflammasome activation in infected macrophages drives COVID-19 pathology.bioRxiv [Preprint]. 2022 Apr 1:2021.09.27.461948. doi: 10.1101/2021.09.27.461948. bioRxiv. 2022. Update in: Nature. 2022 Jun;606(7914):585-593. doi: 10.1038/s41586-022-04802-1. PMID: 34611663 Free PMC article. Updated. Preprint.
References
-
- Pairo-Castineira E et al. Genetic mechanisms of critical illness in Covid-19. Nature, 1–1 (2020). - PubMed
MeSH terms
Substances
Grants and funding
- R01 GM131642/GM/NIGMS NIH HHS/United States
- T32 AI007517/AI/NIAID NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U54 DK106857/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P01 AI061093/AI/NIAID NIH HHS/United States
- F30 CA239444/CA/NCI NIH HHS/United States
- K08 AI128043/AI/NIAID NIH HHS/United States
- U01 CA260507/CA/NCI NIH HHS/United States
- P30 CA016359/CA/NCI NIH HHS/United States
- R01 GM135928/GM/NIGMS NIH HHS/United States
- R35 GM142687/GM/NIGMS NIH HHS/United States
- R01 AI157488/AI/NIAID NIH HHS/United States
- R01 AI118855/AI/NIAID NIH HHS/United States
- R01 AI148467/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

