Vocal tract allometry in a mammalian vocal learner
- PMID: 35483405
- PMCID: PMC9124484
- DOI: 10.1242/jeb.243766
Vocal tract allometry in a mammalian vocal learner
Erratum in
-
Correction: Vocal tract allometry in a mammalian vocal learner.J Exp Biol. 2022 Dec 15;225(24):jeb245305. doi: 10.1242/jeb.245305. Epub 2022 Dec 16. J Exp Biol. 2022. PMID: 36524434 Free PMC article. No abstract available.
Abstract
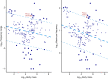
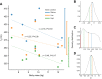
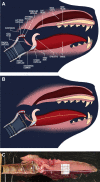
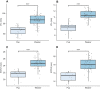
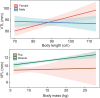
Acoustic allometry occurs when features of animal vocalisations can be predicted from body size measurements. Despite this being considered the norm, allometry sometimes breaks, resulting in species sounding smaller or larger than expected for their size. A recent hypothesis suggests that allometry-breaking mammals cluster into two groups: those with anatomical adaptations to their vocal tracts and those capable of learning new sounds (vocal learners). Here, we tested which mechanism is used to escape from acoustic allometry by probing vocal tract allometry in a proven mammalian vocal learner, the harbour seal (Phoca vitulina). We tested whether vocal tract structures and body size scale allometrically in 68 young individuals. We found that both body length and body mass accurately predict vocal tract length and one tracheal dimension. Independently, body length predicts vocal fold length while body mass predicts a second tracheal dimension. All vocal tract measures are larger in weaners than in pups and some structures are sexually dimorphic within age classes. We conclude that harbour seals do comply with anatomical allometric constraints. However, allometry between body size and vocal fold length seems to emerge after puppyhood, suggesting that ontogeny may modulate the anatomy-learning distinction previously hypothesised as clear cut. We suggest that seals, and perhaps other species producing signals that deviate from those expected from their vocal tract dimensions, may break allometry without morphological adaptations. In seals, and potentially other vocal learning mammals, advanced neural control over vocal organs may be the main mechanism for breaking acoustic allometry.
Keywords: Acoustic allometry; Harbour seal; Larynx; Pinniped; Trachea; Vocal anatomy; Vocal tract.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


References
-
- Akinwande, M. O., Dikko, H. G. and Samson, A. (2015). Variance inflation factor: as a condition for the inclusion of suppressor variable(s) in regression analysis. Open J. Stat. 5, 754. 10.4236/ojs.2015.57075 - DOI
-
- Bowen, W., Oftedal, O., Boness, D. and Iverson, S. (1994). The effect of maternal age and other factors on birth mass in the harbour seal. Can. J. Zool. 72, 8-14. 10.1139/z94-002 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

