Measuring metabolic rate in single flies during sleep and waking states via indirect calorimetry
- PMID: 35483506
- PMCID: PMC9310448
- DOI: 10.1016/j.jneumeth.2022.109606
Measuring metabolic rate in single flies during sleep and waking states via indirect calorimetry
Abstract
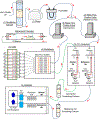
Background: Drosophila melanogaster is a leading genetic model for studying the neural regulation of sleep. Sleep is associated with changes in behavior and physiological state that are largely conserved across species. The investigation of sleep in flies has predominantly focused on behavioral readouts of sleep because physiological measurements, including changes in brain activity and metabolic rate, are less accessible. We have previously used stop-flow indirect calorimetry to measure whole body metabolic rate in single flies and have shown that in flies, like mammals, metabolic rate is reduced during sleep.
New method: Here, we describe a modified version of this system that allows for efficient and highly sensitive acquisition of CO2 output from single flies.
Results: In this modified system, we show that sleep-dependent changes in metabolic rate are diminished in aging flies, supporting the notion that sleep quality is reduced as flies age. We also describe a modification that allows for simultaneous acquisition of CO2 and O2 levels, providing a respiratory quotient that quantifies how metabolic stores are utilized. We find that the respiratory quotient identified in flies on an all-sugar diet is suggestive of lipogenesis, where the dietary sugar provided to the flies is being converted to fat.
Comparison with existing methods and conclusions: Taken together, the measurement of metabolic rate via indirect calorimetry not only provides a physiological readout of sleep depth, but also provides insight the metabolic regulation of nutrient utilization, with broad applications to genetic studies in flies.
Keywords: Aging; CO(2) output; Drosophila; Indirect calorimetry; Metabolism.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
DECLARATIONS OF INTEREST
An author of this manuscript has the following competing interests: JK is employed by Sable Systems International, which designs and sells metabolic systems. The system design and analysis performed here required custom set up and programming. Therefore, we feel that he meets the requirements outlined in the journal for authorship.
Figures



Similar articles
-
Sleep-Dependent Modulation of Metabolic Rate in Drosophila.Sleep. 2017 Aug 1;40(8):zsx084. doi: 10.1093/sleep/zsx084. Sleep. 2017. PMID: 28541527 Free PMC article.
-
Altered regulation of sleep and feeding contributes to starvation resistance in Drosophila melanogaster.J Exp Biol. 2014 Sep 1;217(Pt 17):3122-32. doi: 10.1242/jeb.103309. Epub 2014 Jun 19. J Exp Biol. 2014. PMID: 24948636 Free PMC article.
-
The Regulation of Drosophila Sleep.Curr Biol. 2021 Jan 11;31(1):R38-R49. doi: 10.1016/j.cub.2020.10.082. Curr Biol. 2021. PMID: 33434488 Review.
-
Sleep homeostasis in Drosophila melanogaster.Sleep. 2004 Jun 15;27(4):628-39. doi: 10.1093/sleep/27.4.628. Sleep. 2004. PMID: 15282997
-
Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals.Int J Obes (Lond). 2006 Sep;30(9):1322-31. doi: 10.1038/sj.ijo.0803280. Epub 2006 Jun 27. Int J Obes (Lond). 2006. PMID: 16801931 Review.
Cited by
-
Spenito-dependent metabolic sexual dimorphism intrinsic to fat storage cells.bioRxiv [Preprint]. 2023 Feb 18:2023.02.17.528952. doi: 10.1101/2023.02.17.528952. bioRxiv. 2023. Update in: Genetics. 2023 Nov 1;225(3):iyad164. doi: 10.1093/genetics/iyad164. PMID: 36824729 Free PMC article. Updated. Preprint.
-
Oxygen consumption rate of flatworms under the influence of wake- and sleep-promoting neurotransmitters.J Exp Zool A Ecol Integr Physiol. 2024 Dec;341(10):1130-1136. doi: 10.1002/jez.2828. Epub 2024 May 27. J Exp Zool A Ecol Integr Physiol. 2024. PMID: 38801005 Free PMC article.
-
RNA virus-mediated changes in organismal oxygen consumption rate in young and old Drosophila melanogaster males.Aging (Albany NY). 2023 Mar 22;15(6):1748-1767. doi: 10.18632/aging.204593. Epub 2023 Mar 22. Aging (Albany NY). 2023. PMID: 36947702 Free PMC article.
-
Spenito-dependent metabolic sexual dimorphism intrinsic to fat storage cells.Genetics. 2023 Nov 1;225(3):iyad164. doi: 10.1093/genetics/iyad164. Genetics. 2023. PMID: 37738330 Free PMC article.
-
Integrated Respirometry and Metabolomics Unveil Circadian Metabolic Dynamics in Drosophila.bioRxiv [Preprint]. 2025 Jul 25:2025.07.22.665580. doi: 10.1101/2025.07.22.665580. bioRxiv. 2025. PMID: 40777429 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

