Blocking ribosomal protein S6 phosphorylation inhibits podocyte hypertrophy and focal segmental glomerulosclerosis
- PMID: 35483522
- PMCID: PMC10711420
- DOI: 10.1016/j.kint.2022.02.037
Blocking ribosomal protein S6 phosphorylation inhibits podocyte hypertrophy and focal segmental glomerulosclerosis
Abstract
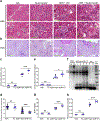
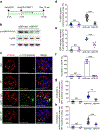
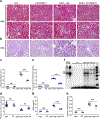
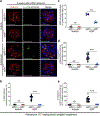
Ribosomal protein S6 (rpS6) phosphorylation mediates the hypertrophic growth of kidney proximal tubule cells. However, the role of rpS6 phosphorylation in podocyte hypertrophy and podocyte loss during the pathogenesis of focal segmental glomerulosclerosis (FSGS) remains undefined. Here, we examined rpS6 phosphorylation levels in kidney biopsy specimens from patients with FSGS and in podocytes from mouse kidneys with Adriamycin-induced FSGS. Using genetic and pharmacologic approaches in the mouse model of FSGS, we investigated the role of rpS6 phosphorylation in podocyte hypertrophy and loss during development and progression of FSGS. Phosphorylated rpS6 was found to be markedly increased in the podocytes of patients with FSGS and Adriamycin-induced FSGS mice. Genetic deletion of the Tuberous sclerosis 1 gene in kidney glomerular podocytes activated mammalian target of rapamycin complex 1 signaling to rpS6 phosphorylation, resulting in podocyte hypertrophy and pathologic features similar to those of patients with FSGS including podocyte loss, leading to segmental glomerulosclerosis. Since protein phosphatase 1 is known to negatively regulate rpS6 phosphorylation, treatment with an inhibitor increased phospho-rpS6 levels, promoted podocyte hypertrophy and exacerbated formation of FSGS lesions. Importantly, blocking rpS6 phosphorylation (either by generating congenic rpS6 knock-in mice expressing non-phosphorylatable rpS6 or by inhibiting ribosomal protein S6 kinase 1-mediated rpS6 phosphorylation with an inhibitor) significantly blunted podocyte hypertrophy, inhibited podocyte loss, and attenuated formation of FSGS lesions. Thus, our study provides genetic and pharmacologic evidence indicating that specifically targeting rpS6 phosphorylation can attenuate the development of FSGS lesions by inhibiting podocyte hypertrophy and associated podocyte depletion.
Keywords: focal segmental glomerulosclerosis (FSGS); podocyte hypertrophy; ribosomal protein S6 (rpS6).
Copyright © 2022 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURE
All the authors declared no competing interests. The abstract of this paper has been selected for presentation at the World Congress of Nephrology 2022 (WCN’22) scheduled for February 24–27, 2022. This abstract would be published electronically in
Figures
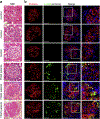
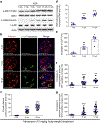
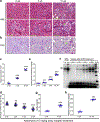
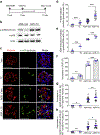
References
-
- Wharram BL, Goyal M, Wiggins JE, et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. - PubMed
-
- Kim YH, Goyal M, Kurnit D, et al. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int. 2001;60:957–968. - PubMed
-
- Matsusaka T, Xin J, Niwa S, et al. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol. 2005;16:1013–1023. - PubMed
-
- Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

