Capmatinib suppresses LPS-induced interaction between HUVECs and THP-1 monocytes through suppression of inflammatory responses
- PMID: 35483573
- PMCID: PMC10267969
- DOI: 10.1016/j.bj.2022.04.005
Capmatinib suppresses LPS-induced interaction between HUVECs and THP-1 monocytes through suppression of inflammatory responses
Abstract
Background: Capmatinib (CAP) is a drug that has been used to treat non-small cell lung cancer (NSCLC) in adults. Presently, its novel effects on skeletal muscle insulin signaling, inflammation, and lipogenesis in adipocytes have been uncovered with a perspective of drug repositioning. However, the impact of CAP on LPS-mediated interaction between human umbilical vein endothelial cells (HUVECs) and THP-1 monocytes has yet to be investigated.
Methods: HUVECs and THP-1 monocytes were treated with LPS and CAP. The protein expression levels were determined using Western blotting. Target protein knockdown was conducted using small interfering (si) RNA transfection. Interactions between HUVECs and THP-1 cells were assayed using green fluorescent dye.
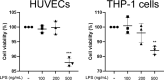
Results: This study found that CAP treatment ameliorated cell adhesion between THP-1 monocytes and HUVECs and the expression of adhesive molecules, such as intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin. Moreover, phosphorylation of inflammatory markers, such as NFκB and IκB as well as TNFα and monocyte chemoattractant protein-1 (MCP-1) released from HUVECs and THP-1 monocytes, was prevented by CAP treatment. Treatment with CAP augmented PPARδ and IL-10 expression. siRNA-associated suppression of PPARδ and IL-10 abolished the effects of CAP on cell interaction between HUVECs and THP-1 cells and inflammatory responses. Further, PPARδ siRNA mitigated CAP-mediated induction of IL-10 expression.
Conclusion: These findings imply that CAP improves inflamed endothelial-monocyte adhesion via a PPARδ/IL-10-dependent pathway. The current study provides in vitro evidence for a therapeutic approach for treating atherosclerosis.
Keywords: Capmatinib; HUVEC; IL-10; Inflammation; PPAR delta; THP-1.
Copyright © 2022 Chang Gung University. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest None.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

