Dapagliflozin and Kidney Outcomes in Hospitalized Patients with COVID-19 Infection: An Analysis of the DARE-19 Randomized Controlled Trial
- PMID: 35483733
- PMCID: PMC9269587
- DOI: 10.2215/CJN.14231021
Dapagliflozin and Kidney Outcomes in Hospitalized Patients with COVID-19 Infection: An Analysis of the DARE-19 Randomized Controlled Trial
Abstract
Background and objectives: Patients who were hospitalized with coronavirus disease 2019 (COVID-19) infection are at high risk of AKI and KRT, especially in the presence of CKD. The Dapagliflozin in Respiratory Failure in Patients with COVID-19 (DARE-19) trial showed that in patients hospitalized with COVID-19, treatment with dapagliflozin versus placebo resulted in numerically fewer participants who experienced organ failure or death, although these differences were not statistically significant. We performed a secondary analysis of the DARE-19 trial to determine the efficacy and safety of dapagliflozin on kidney outcomes in the overall population and in prespecified subgroups of participants defined by baseline eGFR.
Design, setting, participants, & measurements: The DARE-19 trial randomized 1250 patients who were hospitalized (231 [18%] had eGFR <60 ml/min per 1.73 m2) with COVID-19 and cardiometabolic risk factors to dapagliflozin or placebo. Dual primary outcomes (time to new or worsened organ dysfunction or death, and a hierarchical composite end point of recovery [change in clinical status by day 30]), and the key secondary kidney outcome (composite of AKI, KRT, or death), and safety were assessed in participants with baseline eGFR <60 and ≥60 ml/min per 1.73 m2.
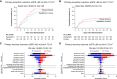
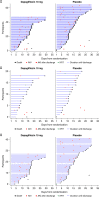
Results: The effect of dapagliflozin versus placebo on the primary prevention outcome (hazard ratio, 0.80; 95% confidence interval, 0.58 to 1.10), primary recovery outcome (win ratio, 1.09; 95% confidence interval, 0.97 to 1.22), and the composite kidney outcome (hazard ratio, 0.74; 95% confidence interval, 0.50 to 1.07) were consistent across eGFR subgroups (P for interaction: 0.98, 0.67, and 0.44, respectively). The effects of dapagliflozin on AKI were also similar in participants with eGFR <60 ml/min per 1.73 m2 (hazard ratio, 0.71; 95% confidence interval, 0.29 to 1.77) and ≥60 ml/min per 1.73 m2 (hazard ratio, 0.69; 95% confidence interval, 0.37 to 1.29). Dapagliflozin was well tolerated in participants with eGFR <60 and ≥60 ml/min per 1.73 m2.
Conclusions: The effects of dapagliflozin on primary and secondary outcomes in hospitalized participants with COVID-19 were consistent in those with eGFR below/above 60 ml/min per 1.73 m2. Dapagliflozin was well tolerated and did not increase the risk of AKI in participants with eGFR below or above 60 ml/min per 1.73 m2.
Keywords: COVID-19; acute kidney injury; cardiovascular disease; chronic kidney disease; diabetes; heart failure; hospitalization; mortality risk; outcomes; randomized controlled trials.
Copyright © 2022 by the American Society of Nephrology.
Figures



Comment in
-
Learnings from Throwing Paint at the Wall for COVID-19 with an SGLT2 Inhibitor.Clin J Am Soc Nephrol. 2022 May;17(5):628-630. doi: 10.2215/CJN.03250322. Epub 2022 Apr 28. Clin J Am Soc Nephrol. 2022. PMID: 35483735 Free PMC article. No abstract available.
References
-
- Tajbakhsh A, Gheibi Hayat SM, Taghizadeh H, Akbari A, Inabadi M, Savardashtaki A, Johnston TP, Sahebkar A: COVID-19 and cardiac injury: Clinical manifestations, biomarkers, mechanisms, diagnosis, treatment, and follow up. Expert Rev Anti Infect Ther 19: 345–357, 2021 - PubMed
-
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B: Factors associated with COVID-19-related death using OpenSAFELY. Nature 584: 430–436, 2020 - PMC - PubMed
-
- Holman N, Knighton P, Kar P, O’Keefe J, Curley M, Weaver A, Barron E, Bakhai C, Khunti K, Wareham NJ, Sattar N, Young B, Valabhji J: Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: A population-based cohort study. Lancet Diabetes Endocrinol 8: 823–833, 2020 - PMC - PubMed
-
- Oliveira CB, Lima CAD, Vajgel G, Campos Coelho AV, Sandrin-Garcia P: High burden of acute kidney injury in COVID-19 pandemic: Systematic review and meta-analysis. J Clin Pathol 74: 796–803, 2021 - PubMed
-
- Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, Sutherland A, Puri S, Srivastava A, Leonberg-Yoo A, Shehata AM, Flythe JE, Rashidi A, Schenck EJ, Goyal N, Hedayati SS, Dy R, Bansal A, Athavale A, Nguyen HB, Vijayan A, Charytan DM, Schulze CE, Joo MJ, Friedman AN, Zhang J, Sosa MA, Judd E, Velez JCQ, Mallappallil M, Redfern RE, Bansal AD, Neyra JA, Liu KD, Renaghan AD, Christov M, Molnar MZ, Sharma S, Kamal O, Boateng JO, Short SAP, Admon AJ, Sise ME, Wang W, Parikh CR, Leaf DE; STOP-COVID Investigators : AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol 32: 161–176, 2021 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

