In situ bioprinting: intraoperative implementation of regenerative medicine
- PMID: 35483990
- PMCID: PMC9481658
- DOI: 10.1016/j.tibtech.2022.03.009
In situ bioprinting: intraoperative implementation of regenerative medicine
Abstract
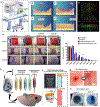
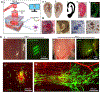
Bioprinting has emerged as a strong tool for devising regenerative therapies to address unmet medical needs. However, the translation of conventional in vitro bioprinting approaches is partially hindered due to challenges associated with the fabrication and implantation of irregularly shaped scaffolds and their limited accessibility for immediate treatment by healthcare providers. An alternative strategy that has recently drawn significant attention is to directly print the bioink into the patient's body, so-called 'in situ bioprinting'. The bioprinting strategy and the associated bioink need to be specifically designed for in situ bioprinting to meet the particular requirements of direct deposition in vivo. In this review, we discuss the developed in situ bioprinting strategies, their advantages, challenges, and possible future improvements.
Keywords: bioinks; bioprinting; handheld printers; in situ printing; robotic bioprinters.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
In Situ Bioprinting: Process, Bioinks, and Applications.ACS Appl Bio Mater. 2024 Dec 16;7(12):7987-8007. doi: 10.1021/acsabm.3c01303. Epub 2024 Apr 10. ACS Appl Bio Mater. 2024. PMID: 38598256 Review.
-
In situ bioprinting - Bioprinting from benchside to bedside?Acta Biomater. 2020 Jan 1;101:14-25. doi: 10.1016/j.actbio.2019.08.045. Epub 2019 Aug 30. Acta Biomater. 2020. PMID: 31476384 Review.
-
From lab to life: advances inin-situbioprinting and bioink technology.Biomed Mater. 2024 Dec 20;20(1). doi: 10.1088/1748-605X/ad9dd0. Biomed Mater. 2024. PMID: 39704234 Review.
-
Handheld bioprinting strategies for in situ wound dressing.Essays Biochem. 2021 Aug 10;65(3):533-543. doi: 10.1042/EBC20200098. Essays Biochem. 2021. PMID: 34028545 Free PMC article. Review.
-
In vivo bioprinting: Broadening the therapeutic horizon for tissue injuries.Bioact Mater. 2023 Feb 6;25:201-222. doi: 10.1016/j.bioactmat.2023.01.018. eCollection 2023 Jul. Bioact Mater. 2023. PMID: 36817820 Free PMC article. Review.
Cited by
-
Additively manufactured porous scaffolds by design for treatment of bone defects.Front Bioeng Biotechnol. 2024 Jan 19;11:1252636. doi: 10.3389/fbioe.2023.1252636. eCollection 2023. Front Bioeng Biotechnol. 2024. PMID: 38312510 Free PMC article. Review.
-
Nano-biomaterials and advanced fabrication techniques for engineering skeletal muscle tissue constructs in regenerative medicine.Nano Converg. 2023 Oct 21;10(1):48. doi: 10.1186/s40580-023-00398-y. Nano Converg. 2023. PMID: 37864632 Free PMC article. Review.
-
3D Bioprinting Strategies for Articular Cartilage Tissue Engineering.Ann Biomed Eng. 2024 Jul;52(7):1883-1893. doi: 10.1007/s10439-023-03236-8. Epub 2023 May 18. Ann Biomed Eng. 2024. PMID: 37204546 Review.
-
Revolutionizing nephrology research: expanding horizons with kidney-on-a-chip and beyond.Front Bioeng Biotechnol. 2024 Mar 28;12:1373386. doi: 10.3389/fbioe.2024.1373386. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38605984 Free PMC article. Review.
-
Intraoperative Creation of Tissue-Engineered Grafts with Minimally Manipulated Cells: New Concept of Bone Tissue Engineering In Situ.Bioengineering (Basel). 2022 Nov 17;9(11):704. doi: 10.3390/bioengineering9110704. Bioengineering (Basel). 2022. PMID: 36421105 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

