The mycobacterial desaturase DesA2 is associated with mycolic acid biosynthesis
- PMID: 35484172
- PMCID: PMC9050676
- DOI: 10.1038/s41598-022-10589-y
The mycobacterial desaturase DesA2 is associated with mycolic acid biosynthesis
Abstract
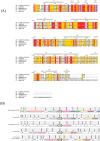
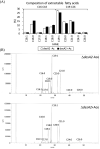
Mycolic acids are critical for the survival and virulence of Mycobacterium tuberculosis, the causative agent of tuberculosis. Double bond formation in the merochain of mycolic acids remains poorly understood, though we have previously shown desA1, encoding an aerobic desaturase, is involved in mycolic acid desaturation. Here we show that a second desaturase encoded by desA2 is also involved in mycolate biosynthesis. DesA2 is essential for growth of the fast-growing Mycobacterium smegmatis in laboratory media. Conditional depletion of DesA2 led to a decrease in mycolic acid biosynthesis and loss of mycobacterial viability. Additionally, DesA2-depleted cells also accumulated fatty acids of chain lengths C19-C24. The complete loss of mycolate biosynthesis following DesA2 depletion, and the absence of any monoenoic derivatives (found to accumulate on depletion of DesA1) suggests an early role for DesA2 in the mycolic acid biosynthesis machinery, highlighting its potential as a drug target.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

