Daily longitudinal sampling of SARS-CoV-2 infection reveals substantial heterogeneity in infectiousness
- PMID: 35484231
- PMCID: PMC9084242
- DOI: 10.1038/s41564-022-01105-z
Daily longitudinal sampling of SARS-CoV-2 infection reveals substantial heterogeneity in infectiousness
Abstract
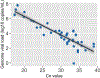
The dynamics of SARS-CoV-2 replication and shedding in humans remain poorly understood. We captured the dynamics of infectious virus and viral RNA shedding during acute infection through daily longitudinal sampling of 60 individuals for up to 14 days. By fitting mechanistic models, we directly estimated viral expansion and clearance rates and overall infectiousness for each individual. Significant person-to-person variation in infectious virus shedding suggests that individual-level heterogeneity in viral dynamics contributes to 'superspreading'. Viral genome loads often peaked days earlier in saliva than in nasal swabs, indicating strong tissue compartmentalization and suggesting that saliva may serve as a superior sampling site for early detection of infection. Viral loads and clearance kinetics of Alpha (B.1.1.7) and previously circulating non-variant-of-concern viruses were mostly indistinguishable, indicating that the enhanced transmissibility of this variant cannot be explained simply by higher viral loads or delayed clearance. These results provide a high-resolution portrait of SARS-CoV-2 infection dynamics and implicate individual-level heterogeneity in infectiousness in superspreading.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures


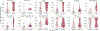
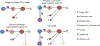
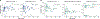





Update of
-
Daily sampling of early SARS-CoV-2 infection reveals substantial heterogeneity in infectiousness.medRxiv [Preprint]. 2021 Jul 12:2021.07.12.21260208. doi: 10.1101/2021.07.12.21260208. medRxiv. 2021. Update in: Nat Microbiol. 2022 May;7(5):640-652. doi: 10.1038/s41564-022-01105-z. PMID: 34282424 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

