Lipid metabolism in T cell signaling and function
- PMID: 35484263
- PMCID: PMC11103273
- DOI: 10.1038/s41589-022-01017-3
Lipid metabolism in T cell signaling and function
Abstract
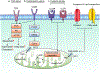
T cells orchestrate adaptive immunity against pathogens and other immune challenges, but their dysfunction can also mediate the pathogenesis of cancer and autoimmunity. Metabolic adaptation in response to immunological and microenvironmental signals contributes to T cell function and fate decision. Lipid metabolism has emerged as a key regulator of T cell responses, with selective lipid metabolites serving as metabolic rheostats to integrate environmental cues and interplay with intracellular signaling processes. Here, we discuss how extracellular, de novo synthesized and membrane lipids orchestrate T cell biology. We also describe the roles of lipids as regulators of intracellular signaling at the levels of transcriptional, epigenetic and post-translational regulation in T cells. Finally, we summarize therapeutic targeting of lipid metabolism and signaling, and conclude with a discussion of important future directions. Understanding the molecular and functional interplay between lipid metabolism and T cell biology will ultimately inform therapeutic intervention for human disease.
© 2022. Springer Nature America, Inc.
Conflict of interest statement
Competing interests
H.C. is a consultant for Kumquat Biosciences.
Figures




References
-
- Dudek M et al. Auto-aggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature 592, 444–449, doi: 10.1038/s41586-021-03233-8 (2021). - DOI - PubMed
-
This paper shows a pathogenic role for the SCFA acetate in CD8+ T cell-mediated autoinflammation and tissue damage in the liver, which is in contrast to the protective roles for SCFAs in tissue homeostasis or anti-pathogen immunity.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

