A randomised study to assess the nicotine pharmacokinetics of an oral nicotine pouch and two nicotine replacement therapy products
- PMID: 35484309
- PMCID: PMC9050656
- DOI: 10.1038/s41598-022-10544-x
A randomised study to assess the nicotine pharmacokinetics of an oral nicotine pouch and two nicotine replacement therapy products
Abstract
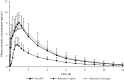
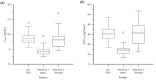
Nicotine replacement therapies (NRTs) are intended for short-term use to help cigarette smokers to quit. Some smokers find NRTs ineffective or seek a more satisfactory source of nicotine. Tobacco-free oral nicotine pouch (NP) products have emerged as a potential reduced risk product compared with cigarettes and other tobacco products. In a randomised crossover clinical study, thirty-four healthy adult smokers were enrolled and their nicotine Cmax and AUC0-T determined for three 4 mg nicotine products (NP, gum, lozenge) under fasting conditions. The NP, lozenge and gum mean Cmax values were 8.5, 8.3 and 4.4 ng/mL, AUC0-T values were 30.6, 31.5 and 14.3 ng*h/mL, respectively. The NP showed similar nicotine bioavailability to the lozenge (p = 0.6526 (Cmax), p = 1.0000 (AUC0-T)), and superior bioavailability to the gum (p < 0.0001 for Cmax and AUC0-T). Compared with the lozenge, the NP demonstrated greater product satisfaction with a higher number of positive responses to subjective satisfaction questions. All products were judged to be well-tolerated; the incidence of minor adverse events was lower for the NP (18.2%) than the lozenge (33.3%) or gum (18.8%). In summary, NPs may provide smokers with a more satisfying alternative nicotine source as compared to the reference NRTs.Study Registry/Registered Trial No: ISRCTN/ISRCTN65708311.
© 2022. The Author(s).
Conflict of interest statement
Imperial Tobacco Canada (ITC) funded this study. ITC is a subsidiary of BAT, a company that manufactures tobacco and nicotine products. All authors are or were employees of BAT, ITC or R.J. Reynolds Tobacco Company, a wholly-owned subsidiary of BAT.
Figures

References
-
- US Department of Health and Human Services. The health consequences of smoking–50 years of progress: A report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention. Available from: https://www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/inde... [Accessed 01 February 2021] (2014). - PubMed
-
- Rodgman A, Perfetti TA. The chemical components of tobacco and tobacco smoke. 2. CRC Press; 2013.
-
- Gottlieb, S. Protecting American families: Comprehensive approach to nicotine and tobacco. Speech by Scott Gottlieb, FDA, White Oak, MD. Available from: https://www.fda.gov/news-events/speeches-fda-officials/protecting-americ... [Accessed 01 February 2021] (2017).
-
- Royal College of Physicians. Nicotine with smoke: Tobacco harm reduction. London: RCP. Available from: https://www.rcplondon.ac.uk/projects/outputs/nicotine-without-smoke-toba... [Accessed 01 February 2021] (2016).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

