Chronic thromboembolic pulmonary hypertension and impairment after pulmonary embolism: the FOCUS study
- PMID: 35484821
- PMCID: PMC9492241
- DOI: 10.1093/eurheartj/ehac206
Chronic thromboembolic pulmonary hypertension and impairment after pulmonary embolism: the FOCUS study
Abstract
Aims: To systematically assess late outcomes of acute pulmonary embolism (PE) and to investigate the clinical implications of post-PE impairment (PPEI) fulfilling prospectively defined criteria.
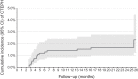
Methods and results: A prospective multicentre observational cohort study was conducted in 17 large-volume centres across Germany. Adult consecutive patients with confirmed acute symptomatic PE were followed with a standardized assessment plan and pre-defined visits at 3, 12, and 24 months. The co-primary outcomes were (i) diagnosis of chronic thromboembolic pulmonary hypertension (CTEPH), and (ii) PPEI, a combination of persistent or worsening clinical, functional, biochemical, and imaging parameters during follow-up. A total of 1017 patients (45% women, median age 64 years) were included in the primary analysis. They were followed for a median duration of 732 days after PE diagnosis. The CTEPH was diagnosed in 16 (1.6%) patients, after a median of 129 days; the estimated 2-year cumulative incidence was 2.3% (1.2-4.4%). Overall, 880 patients were evaluable for PPEI; the 2-year cumulative incidence was 16.0% (95% confidence interval 12.8-20.8%). The PPEI helped to identify 15 of the 16 patients diagnosed with CTEPH during follow-up (hazard ratio for CTEPH vs. no CTEPH 393; 95% confidence interval 73-2119). Patients with PPEI had a higher risk of re-hospitalization and death as well as worse quality of life compared with those without PPEI.
Conclusion: In this prospective study, the cumulative 2-year incidence of CTEPH was 2.3%, but PPEI diagnosed by standardized criteria was frequent. Our findings support systematic follow-up of patients after acute PE and may help to optimize guideline recommendations and algorithms for post-PE care.
Keywords: Chronic thromboembolic pulmonary hypertension; Follow-up; Functional impairment; Pulmonary embolism.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Cardiology.
Conflict of interest statement
Conflict of interest: A.C.M. was supported by a research grant from the German Academic Exchange Service (DAAD; 57440918). S.B. reports grants or contracts from Bayer, INARI, Boston Scientific, Medtronic, Bard, SANOFI, and Concept Medical; consulting fees from INARI; payment or honoraria from INARI, Boston Scientific, and Concept Medical; and support for attending meetings and/or travel from Bayer and Daiichi Sankyo. R.E. reports consulting fees from Actelion Germany, Boehringer Ingelheim, Janssen, AstraZeneca, OMT, and LungPacer; payment or honoraria from Janssen, Boehringer Ingelheim, AstraZeneca, OMT, Berlin Chemie, and Novartis; support for attending meetings and/or travel from Janssen, Boehringer Ingelheim, AstraZeneca, OMT, Berlin Chemie, and Novartis; and participation on a Data Safety Monitoring Board or Advisory Board for Janssen, Boehringer Ingelheim, AstraZeneca, and Berlin Chemie. M.F. reports payment or honoraria and support for attending meetings and/or travel from Janssen. F.G. reports grants or contracts from Janssen, Bayer; consulting fees from Bayer & Janssen and AstraZeneca; payment or honoraria from Bayer, Janssen, AstraZeneca, and MSD; payment for expert testimony from Janssen; and receipt of equipment, materials, drugs, medical writing, gifts, or other services from Janssen. H.-A.G. reports grants or contracts from Actelion/Janssen and Bayer; consulting fees from Actelion/Janssen, Bayer, MSD, Accelleron, MorphogenIX, and Gossamer Bio; payment or honoraria from Actelion/Janssen, Bayer, MSD, and Gossamer Bio; and support for attending meetings and/or travel from Actelion/Janssen, Bayer, and MSD. A.G. reports payment or honoraria from Bayer Vital, Roche, MSD, and Boehringer-Ingelheim; and support for attending meetings and/or travel from Bayer Vital. E.G. reports consulting fees from Actelion, Bayer/MSD, GSK, United Therapeutics, Novartis, REATA, OMT, and Pfizer; payment or honoraria from Actelion, Bayer/MSD, and GSK; participation on a Data Safety Monitoring Board or Advisory Board for MSD, Janssen and Bayer; and leadership or fiduciary role for Actelion/Janssen. M.Ha. reports consulting fees from MSD; payment or honoraria from Actelion, AstraZeneca, Bayer, Berlin Chemie, Janssen-Cilag, and MSD; support for attending meetings and/or travel from Actelion; and participation on a Data Safety Monitoring Board or Advisory Board for Acceleron, Actelion, GSK, Janssen-Cilag, and MSD. M.He. reports consulting fees from Actelion, Bayer, Berlin Chemie, BMS, Boehringer Ingelheim, Janssen, MSD, and Pfizer; payment or honoraria from Actelion, AstraZeneca, Bayer, BMS, Berlin Chemie, Boehringer Ingelheim, Daiichi Sankyo, Janssen, MSD, and Pfuter Santis; and support for attending meetings and/or travel from Boehringer Ingelheim and Janssen. L.H. reports payment or honoraria from Actelion and MSD. M.M.H. reports consulting fees from Acceleron, Actelion, Bayer, GSK, Janssen, MSD, and Pfizer; and payment or honoraria from Actelion, Bayer, GSK, Janssen, MSD, and Pfizer. F.A.K. reports grants or contracts from Bayer, BMS, Boehringer Ingelheim, MSD, Daiichi Sankyo, Actelion. M.L. reports grants or contracts from Thermo Fisher Scientific, payment or honoraria from Actelion/Johnson & Johnson, Bayer, Daiichi Sankyo, MSD, Pfizer, and Georg Thieme Verlag Stuttgart Germany; support for attending meetings and/or travel from Actelion/Johnson & Johnson and Bayer; participation on a Data Safety Monitoring Board or Advisory Board for Thermo Fisher Scientific. H.H.L. reports travel fees for the study by Bayer, Germany, consulting fees, payment or honoraria, and payment for expert testimony from MSD, Germany; and participation on a Data Safety Monitoring Board or Advisory Board for MSD, Germany. E.M. reports payment or honoraria from Actelion/Janssen, MSD, and Bayer; support for attending meetings and/or travel from Actelion/Janssen, MSD, and Bayer; and participation on a Data Safety Monitoring Board or Advisory Board for Actelion/Janssen. H.-J.S. reports payment or honoraria from Janssen/Actelion, Bayer, GSK, and MSD; and participation on a Data Safety Monitoring Board or Advisory Board for Janssen. R.W. reports grants or contracts from Boehringer Ingelheim and Medtronic; and payment or honoraria from Abbott, AstraZeneca, Bayer, BMS, CVRx, Daichii Sankyo, Novartis, Pfizer, Pharmacosmos, Sanofi, Servier, and SOBI. H.W. reports payment or honoraria from Actelion/Janssen, Bayer, Biotest, Boehringer Ingelheim, MSD, Pfizer, and Roche; support for attending meetings and/or travel from Actelion and Boehringer Ingelheim; and participation on a Data Safety Monitoring Board or Advisory Board for Actelion/Janssen, Boehringer Ingelheim, and MSD. P.S.W. reports study grants from Bayer AG, grants or contracts from Boehringer Ingelheim, DiaSorin, Sanofi-Aventis, AstraZeneca, Bayer Health Care, Bayer Vital, Daiichy Sankyo Europe, and Novartis Pharma; payment or honoraria from Bayer Health Care, Pfizer Pharma, and Bristol Myers Squibb; and other non-financial support from Philips Medical Systems, DiaSorin, and IEM. S.V.K. reports grants or contracts from Bayer AG; consulting fees from Bayer AG, Daiichi Sankyo, and Boston Scientific; and payment or honoraria from Bayer AG, INARI Medical, MSD, Pfizer, and Bristol-Myers Squibb. S.R. reports grants or contracts from Actelion, AstraZeneca, Bayer, Janssen, and Novartis; consulting fees from Abbott, Acceleron, Actelion, Bayer, Janssen, MSD, Novartis, Pfizer, United Therapeutics, and Vifor; payment or honoraria from Actelion, Bayer, BMS, Ferrer, GSK, Janssen, MSD, Novartis, Pfizer, United Therapeutics, and Vifor. The remaining authors (A.C.M., C.A., D.B., L.B., D.F., N.M., F.J.M., C.N., C.O., and K.-H.S.) declare no conflict of interest.
Figures


Comment in
-
FOCUS on sequelae of acute pulmonary embolism: does it pay off?Eur Heart J. 2022 Sep 21;43(36):3399-3401. doi: 10.1093/eurheartj/ehac170. Eur Heart J. 2022. PMID: 35511503 No abstract available.
References
-
- Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543–603. - PubMed
-
- Becattini C, Agnelli G, Lankeit M, Masotti L, Pruszczyk P, Casazza F, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J 2016;48:780–786. - PubMed
-
- Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402–1411. - PubMed
-
- Mai V, Guay CA, Perreault L, Bonnet S, Bertoletti L, Lacasse Y, et al. Extended anticoagulation for VTE: a systematic review and meta-analysis. Chest 2019;155:1199–1216. - PubMed

