An intranasal lentiviral booster reinforces the waning mRNA vaccine-induced SARS-CoV-2 immunity that it targets to lung mucosa
- PMID: 35484842
- PMCID: PMC9044714
- DOI: 10.1016/j.ymthe.2022.04.016
An intranasal lentiviral booster reinforces the waning mRNA vaccine-induced SARS-CoV-2 immunity that it targets to lung mucosa
Abstract
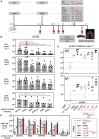
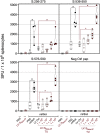
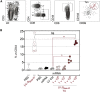
As the coronavirus disease 2019 (COVID-19) pandemic continues and new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern emerge, the adaptive immunity initially induced by the first-generation COVID-19 vaccines starts waning and needs to be strengthened and broadened in specificity. Vaccination by the nasal route induces mucosal, humoral, and cellular immunity at the entry point of SARS-CoV-2 into the host organism and has been shown to be the most effective for reducing viral transmission. The lentiviral vaccination vector (LV) is particularly suitable for this route of immunization owing to its non-cytopathic, non-replicative, and scarcely inflammatory properties. Here, to set up an optimized cross-protective intranasal booster against COVID-19, we generated an LV encoding stabilized spike of SARS-CoV-2 Beta variant (LV::SBeta-2P). mRNA vaccine-primed and -boosted mice, with waning primary humoral immunity at 4 months after vaccination, were boosted intranasally with LV::SBeta-2P. A strong boost effect was detected on cross-sero-neutralizing activity and systemic T cell immunity. In addition, mucosal anti-spike IgG and IgA, lung-resident B cells, and effector memory and resident T cells were efficiently induced, correlating with complete pulmonary protection against the SARS-CoV-2 Delta variant, demonstrating the suitability of the LV::SBeta-2P vaccine candidate as an intranasal booster against COVID-19. LV::SBeta-2P vaccination was also fully protective against Omicron infection of the lungs and central nervous system, in the highly susceptible B6.K18-hACE2IP-THV transgenic mice.
Keywords: SARS-CoV-2 emerging variants of concern; intranasal vaccination; lentiviral vaccine; mucosal booster vaccine; mucosal immunity; waning anti-COVID-19 immunity.
Copyright © 2022 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests P.C. is the founder and CSO of TheraVectys. B.V., A.N., P.A., I.F., F.L.C., F.M., K.N., and F.A. are employees of TheraVectys. L.M. has a consultancy activity for TheraVectys. The other authors declare no competing interests. P.A., I.F., J.L., B.V., F.A., M.B., L.M., and P.C. are the inventors of pending patents directed to the potential of i.n. LV::S vaccination against SARS-CoV-2.
Figures






References
-
- Ku M.W., Bourgine M., Authie P., Lopez J., Nemirov K., Moncoq F., Noirat A., Vesin B., Nevo F., Blanc C., et al. Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models. Cell Host Microbe. 2021;29:236–249.e6. doi: 10.1016/j.chom.2020.12.010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

