Postpartum women's attitudes to disclosure of adult-onset conditions in pregnancy
- PMID: 35484937
- PMCID: PMC9539988
- DOI: 10.1002/pd.6162
Postpartum women's attitudes to disclosure of adult-onset conditions in pregnancy
Abstract
Background: Advanced prenatal genomic technologies can identify risks for adult-onset (AO) conditions in the fetus, challenging the traditional purpose of prenatal testing. Professional guidelines commonly support disclosure of high-penetrance AO actionable conditions, yet attitudes of women/parents to these findings and factors affecting their attitudes are understudied.
Methods: We explored 941 (77% response rate) postpartum women's attitudes towards receiving prenatal genetic information, and associations of sociodemographic, medical and psychological characteristics with their choices, focusing on AO conditions.
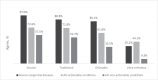
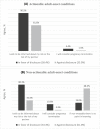
Results: Women largely support the disclosure of actionable AO findings (58.4%), in line with professional guidelines. A third of the women also supported the disclosure of non-actionable AO conditions. Stronger religious observance (p < 0.001) and higher psychological distress (p = 0.024) were associated with decreased interest in receiving actionable AO conditions, whereas higher concern for fetal health yielded increased interest (p = 0.032). Attitudes towards disclosure were strongly associated with women's perceived benefit of such information for their own, partner's, and future child's health. Termination of pregnancy based on such information received very little support.
Conclusion: In-light of the demonstrated understanding of nuanced genetic information and the observed diversity in attitudes, a culturally competent opt-in/out policy could be considered. If full-disclosure is practiced, support should be provided to those expressing higher levels of distress.
© 2022 The Authors. Prenatal Diagnosis published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise, in the writing of this manuscript.
Figures

References
-
- Srebniak MI, Joosten M, Knapen MFCM, et al. Frequency of submicroscopic chromosomal aberrations in pregnancies without increased risk for structural chromosomal aberrations: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2018;51(4):445‐452. 10.1097/01.ogx.0000546163.62393.50 - DOI - PubMed
-
- Gardiner C, Wellesley D, Kilby MD, et al. Recommendations for the Use of Chromosome Microarray in Pregnancy; 2015. https://www.rcpath.org/uploads/assets/06664c28‐0f90‐4230‐86158c91fea14be.... Accessed February, 2022.
MeSH terms
LinkOut - more resources
Full Text Sources

