A novel EHD1/CD44/Hippo/SP1 positive feedback loop potentiates stemness and metastasis in lung adenocarcinoma
- PMID: 35485206
- PMCID: PMC9786223
- DOI: 10.1002/ctm2.836
A novel EHD1/CD44/Hippo/SP1 positive feedback loop potentiates stemness and metastasis in lung adenocarcinoma
Abstract
Background: There is growing evidence that endocytosis plays a pivotal role in cancer metastasis. In this study, we first identified endocytic and metastasis-associated genes (EMGs) and then investigated the biological functions and mechanisms of EMGs.
Methods: Cancer stem cells (CSCs)-like characteristics were evaluated by tumour limiting dilution assays, three-dimensional (3D) spheroid cancer models. Microarray analysis was used to identify the pathways significantly regulated by mammalian Eps15 homology domain protein 1 (EHD1) knockdown. Mass spectrometry (MS) was performed to identify EHD1-interacting proteins. The function of EHD1 as a regulator of cluster of differentiation 44 (CD44) endocytic recycling and lysosomal degradation was determined by CD44 biotinylation and recycling assays.
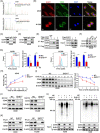
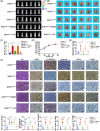
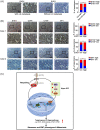
Results: EHD1 was identified as a significant EMG. Knockdown of EHD1 suppressed CSCs-like characteristics, epithelial-mesenchymal transition (EMT), migration and invasion of lung adenocarcinoma (LUAD) cells by increasing Hippo kinase cascade activation. Conversely, EHD1 overexpression inhibited the Hippo pathway to promote cancer stemness and metastasis. Notably, utilising MS analysis, the CD44 protein was identified as a potential binding partner of EHD1. Furthermore, EHD1 enhanced CD44 recycling and stability. Indeed, silencing of CD44 or disruption of the EHD1/CD44 interaction enhanced Hippo pathway activity and reduced CSCs-like traits, EMT and metastasis. Interestingly, specificity protein 1 (SP1), a known downstream target gene of the Hippo-TEA-domain family members 1 (TEAD1) pathway, was found to directly bind to the EHD1 promoter region and induce its expression. Among clinical specimens, the EHD1 expression level in LUAD tissues of metastatic patients was higher than that of non-metastatic patients.
Conclusions: Our findings emphasise that EHD1 might be a potent anti-metastatic target and present a novel regulatory mechanism by which the EHD1/CD44/Hippo/SP1 positive feedback circuit plays pivotal roles in coupling modules of CSCs-like properties and EMT in LUAD. Targeting this loop may serve as a remedy for patients with advanced metastatic LUAD.
Keywords: EHD1; LUAD; hippo signalling pathway; metastasis; stemness.
© 2022 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Figures






References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. - PubMed
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7‐33. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7‐30. - PubMed
-
- Lortet‐Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014;84(1):13‐22. - PubMed
-
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243‐1260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
