Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies
- PMID: 35485439
- PMCID: PMC9052415
- DOI: 10.1093/stcltm/szac004
Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies
Abstract
Aging is a multifaceted and complicated process, manifested by a decline of normal physiological functions across tissues and organs, leading to overt frailty, mortality, and chronic diseases, such as skeletal, cardiovascular, and cognitive disorders, necessitating the development of practical therapeutic approaches. Stem cell aging is one of the leading theories of organismal aging. For decades, mesenchymal stem/stromal cells (MSCs) have been regarded as a viable and ideal source for stem cell-based therapy in anti-aging treatment due to their outstanding clinical characteristics, including easy accessibility, simplicity of isolation, self-renewal and proliferation ability, multilineage differentiation potentials, and immunomodulatory effects. Nonetheless, as evidenced in numerous studies, MSCs undergo functional deterioration and gradually lose stemness with systematic age in vivo or extended culture in vitro, limiting their therapeutic applications. Even though our understanding of the processes behind MSC senescence remains unclear, significant progress has been achieved in elucidating the aspects of the age-related MSC phenotypic changes and possible mechanisms driving MSC senescence. In this review, we aim to summarize the current knowledge of the morphological, biological, and stem-cell marker alterations of aging MSCs, the cellular and molecular mechanisms that underlie MSC senescence, the recent progress made regarding the innovative techniques to rejuvenate senescent MSCs and combat aging, with a particular focus on the interplay between aging MSCs and their niche as well as clinical translational relevance. Also, we provide some promising and novel directions for future research concerning MSC senescence.
Keywords: cell signaling; mesenchymal stem/stromal cells; mitochondrial dysfunction; reactive oxygen species (ROS); rejuvenation; senescence; stem cell niche.
© The Author(s) 2022. Published by Oxford University Press.
Figures


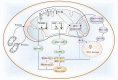
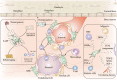
References
-
- Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230-247. - PubMed
-
- Musina RA, Bekchanova ES, Sukhikh GT. Comparison of mesenchymal stem cells obtained from different human tissues. Bull Exp Biol Med. 2005;139(4):504-509. - PubMed
-
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317. - PubMed
-
- Mareschi K, Ferrero I, Rustichelli D, et al. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006;97(4):744-754. - PubMed

