Recovered grasping performance after stroke depends on interhemispheric frontoparietal connectivity
- PMID: 35485480
- PMCID: PMC9976969
- DOI: 10.1093/brain/awac157
Recovered grasping performance after stroke depends on interhemispheric frontoparietal connectivity
Abstract
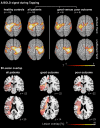
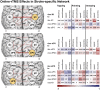
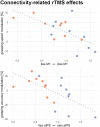
Activity changes in the ipsi- and contralesional parietal cortex and abnormal interhemispheric connectivity between these regions are commonly observed after stroke, however, their significance for motor recovery remains poorly understood. We here assessed the contribution of ipsilesional and contralesional anterior intraparietal cortex (aIPS) for hand motor function in 18 recovered chronic stroke patients and 18 healthy control subjects using a multimodal assessment consisting of resting-state functional MRI, motor task functional MRI, online-repetitive transcranial magnetic stimulation (rTMS) interference, and 3D movement kinematics. Effects were compared against two control stimulation sites, i.e. contralesional M1 and a sham stimulation condition. We found that patients with good motor outcome compared to patients with more substantial residual deficits featured increased resting-state connectivity between ipsilesional aIPS and contralesional aIPS as well as between ipsilesional aIPS and dorsal premotor cortex. Moreover, interhemispheric connectivity between ipsilesional M1 and contralesional M1 as well as ipsilesional aIPS and contralesional M1 correlated with better motor performance across tasks. TMS interference at individual aIPS and M1 coordinates led to differential effects depending on the motor task that was tested, i.e. index finger-tapping, rapid pointing movements, or a reach-grasp-lift task. Interfering with contralesional aIPS deteriorated the accuracy of grasping, especially in patients featuring higher connectivity between ipsi- and contralesional aIPS. In contrast, interference with the contralesional M1 led to impaired grasping speed in patients featuring higher connectivity between bilateral M1. These findings suggest differential roles of contralesional M1 and aIPS for distinct aspects of recovered hand motor function, depending on the reorganization of interhemispheric connectivity.
Keywords: intraparietal sulcus; kinematics; motor recovery; rTMS; resting-state fMRI.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



Comment in
-
Grasping the opportunity: better behavioural diagnoses will lead to better treatments for stroke.Brain. 2023 Mar 1;146(3):799-800. doi: 10.1093/brain/awad001. Brain. 2023. PMID: 36624994 No abstract available.
References
-
- Carod-Artal J, Egido JA, González JL, de Seijas E V. Quality of life among stroke survivors evaluated 1 year after stroke: experience of a stroke unit. Stroke. 2000;31:2995–3000. - PubMed
-
- Rehme AK, Eickhoff SB, Rottschy C, Fink GR, Grefkes C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. NeuroImage. 2012;59:2771–2782. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

