The Impact of Direct-Acting Antiviral Agents on Cytomegalovirus Reactivation in Chronic Hepatitis C Infection
- PMID: 35485698
- PMCID: PMC9375591
- DOI: 10.31557/APJCP.2022.23.4.1365
The Impact of Direct-Acting Antiviral Agents on Cytomegalovirus Reactivation in Chronic Hepatitis C Infection
Abstract
Objective: The co-infection of HCV/CMV may accelerate the progression of liver diseases and worsen responsiveness to IFN treatment. The Direct-acting antiviral agents (DAAs), currently approved therapy for HCV, may cause a transient change in immune status, favoring the reactivation of other viruses. The current study aims to evaluate the impact of DAAs treatment on the reactivation of latent CMV in HCV patients.
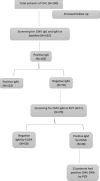
Methods: The serological IgG, IgM Abs against CMV were detected by ELISA on192 HCV patients. The seronegative CMV IgM patients received (sofosbuvir/daclatasvir) regimen, then the CMV reactivation was examined by measuring the CMV IgM by ELISA and CMV DNA by real-time PCR.
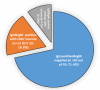
Results: The serological data revealed that all patients were positive for CMV IgG (100%) while (64%) patients were positive for CMV IgM. The seronegative CMV IgM (36%) received the DAAs protocol. The sustained virological response was monitored by measuring the HCV RNA viremia in the patient sera. The serological data revealed that 28.6% of patients had a reactivation of CMV, while 18.5% of patients had detectable CMV DNA viremia. Moreover, there was a significant improvement in liver function as well as a decrease in FIB-4 and APRI scores at EOT. SVR was reached 97.4% among the total studied patients (N= 192).
Conclusion: CMV co-infection has no impact on the response rate to DAAs. However, the CMV reactivation might have occurred after the complete eradication of HCV by DAAs.
Keywords: Direct-acting antiviral; Hepatitis C Virus; Herpes virus; Reactivation.
Conflict of interest statement
No competing financial interests exist.
Figures
Similar articles
-
IL28B polymorphism and cytomegalovirus predict response to treatment in Egyptian HCV type 4 patients.World J Gastroenterol. 2013 Jan 14;19(2):290-8. doi: 10.3748/wjg.v19.i2.290. World J Gastroenterol. 2013. PMID: 23345953 Free PMC article.
-
Direct-acting antiviral treatment in adults infected with hepatitis C virus: Reactivation of hepatitis B virus coinfection as a further challenge.J Clin Virol. 2016 May;78:27-30. doi: 10.1016/j.jcv.2016.02.026. Epub 2016 Mar 3. J Clin Virol. 2016. PMID: 26967675
-
Human cytomegalovirus infection inhibits response of chronic hepatitis-C-virus-infected patients to interferon-based therapy.J Gastroenterol Hepatol. 2011 Jan;26(1):55-62. doi: 10.1111/j.1440-1746.2010.06319.x. J Gastroenterol Hepatol. 2011. PMID: 21175794
-
Hepatitis B Virus Reactivation Associated With Direct-Acting Antiviral Therapy for Chronic Hepatitis C Virus: A Review of Cases Reported to the U.S. Food and Drug Administration Adverse Event Reporting System.Ann Intern Med. 2017 Jun 6;166(11):792-798. doi: 10.7326/M17-0377. Epub 2017 Apr 25. Ann Intern Med. 2017. PMID: 28437794 Review.
-
Reactivation of occult HBV infection in an HIV/HCV Co-infected patient successfully treated with sofosbuvir/ledipasvir: a case report and review of the literature.BMC Infect Dis. 2017 Mar 1;17(1):182. doi: 10.1186/s12879-017-2287-y. BMC Infect Dis. 2017. PMID: 28249574 Free PMC article. Review.
References
-
- Asselah T, Reesink H, Gerstoft J, et al. Efficacy of elbasvir and grazoprevir in participants with hepatitis C virus genotype 4 infection: A pooled analysis. Liver Int. 2018;38:1583–91. - PubMed
-
- Bader el-Din NG, Abd el-Meguid M, Tabll AA, et al. Human cytomegalovirus infection inhibits response of chronic hepatitis-C-virus-infected patients to interferon-based therapy. J Gastroenterol Hepatol. 2011;26:55–62. - PubMed
-
- Breban R, Arafa N, Leroy S, et al. Effect of preventive and curative interventions on hepatitis C virus transmission in Egypt (ANRS 1211): a modelling study. Lancet Glob Health. 2014;2:541–9. - PubMed

