Is there a role for HSF1 in viral infections?
- PMID: 35485710
- PMCID: PMC9157408
- DOI: 10.1002/2211-5463.13419
Is there a role for HSF1 in viral infections?
Abstract
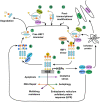
Cells undergo numerous processes to adapt to new challenging conditions and stressors. Heat stress is regulated by a family of heat shock factors (HSFs) that initiate a heat shock response by upregulating the expression of heat shock proteins (HSPs) intended to counteract cellular damage elicited by increased environmental temperature. Heat shock factor 1 (HSF1) is known as the master regulator of the heat shock response and upon its activation induces the transcription of genes that encode for molecular chaperones, such as HSP40, HSP70, and HSP90. Importantly, an accumulating body of studies relates HSF1 with viral infections; the induction of fever during viral infection may activate HSF1 and trigger a consequent heat shock response. Here, we review the role of HSF1 in different viral infections and its impact on the health outcome for the host. Studying the relationship between HSF1 and viruses could open new potential therapeutic strategies given the availability of drugs that regulate the activation of this transcription factor.
Keywords: HSF1; heat shock; heat shock factor 1; stress; viral infections.
© 2022 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pirkkala L, Nykänen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15:1118–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

