IL-20 promotes cutaneous inflammation and peripheral itch sensation in atopic dermatitis
- PMID: 35486004
- PMCID: PMC9321592
- DOI: 10.1096/fj.202101800R
IL-20 promotes cutaneous inflammation and peripheral itch sensation in atopic dermatitis
Abstract
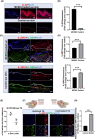
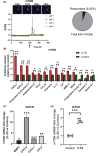
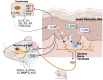
Atopic dermatitis (AD) is a chronic skin disease, which is associated with intense itch, skin barrier dysfunction and eczematous lesions. Aberrant IL-20 expression has been implicated in numerous inflammatory diseases, including psoriasis. However, the role of IL-20 in AD remains unknown. Here, RNA-seq, Q-PCR, and immunocytochemistry were utilized to examine disease-driven changes of IL-20 and its cognate receptor subunits in skin from healthy human subjects, AD patients and murine AD-models. Calcium imaging, knockdown and cytokine array were used to investigate IL-20-evoked responses in keratinocytes and sensory neurons. The murine cheek model and behavioral scoring were employed to evaluate IL-20-elicited sensations in vivo. We found that transcripts and protein of IL-20 were upregulated in skin from human AD and murine AD-like models. Topical MC903 treatment in mice ear enhanced IL-20R1 expression in the trigeminal sensory ganglia, suggesting a lesion-associated and epidermal-driven mechanism for sensitization of sensory IL-20 signaling. IL-20 triggered calcium influx in both keratinocytes and sensory neurons, and promoted their AD-related molecule release and transcription of itch-related genes. In sensory neurons, IL-20 application increased TLR2 transcripts, implicating a link between innate immune response and IL-20. In a murine cheek model of acute itch, intradermal injection IL-20 and IL-13 elicited significant itch-like behavior, though only when co-injected. Our findings provide novel insights into IL-20 function in peripheral (skin-derived) itch and clinically relevant intercellular neuron-epidermal communication, highlighting a role of IL-20 signaling in the pathophysiology of AD, thus forming a new basis for the development of a novel antipruritic strategy via interrupting IL-20 epidermal pathways.
Keywords: IL-13; IL-13Rα1; IL-13Rα2; IL-20; IL-20R1; IL-20R2; atopic dermatitis; cytokine; toll-like receptor.
© 2022 Federation of American Societies for Experimental Biology.
Figures



References
-
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345‐360. - PubMed
-
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483‐1494. - PubMed
-
- Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1. - PubMed
-
- Yosipovitch G. Introduction for understanding and treating itch. Dermatol Ther. 2013;26:83. - PubMed
-
- Ikoma A. [Therapeutic agents of today and the future for atopic dermatitis]. Nihon Yakurigaku Zasshi. 2006;128:411‐415. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

