Midostaurin plus intensive chemotherapy for younger and older patients with AML and FLT3 internal tandem duplications
- PMID: 35486475
- PMCID: PMC9631686
- DOI: 10.1182/bloodadvances.2022007223
Midostaurin plus intensive chemotherapy for younger and older patients with AML and FLT3 internal tandem duplications
Abstract
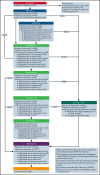
We conducted a single-arm, phase 2 trial (German-Austrian Acute Myeloid Leukemia Study Group [AMLSG] 16-10) to evaluate midostaurin with intensive chemotherapy followed by allogeneic hematopoietic-cell transplantation (HCT) and a 1-year midosta urin maintenance therapy in adult patients with acute myeloid leukemia (AML) and fms-related tyrosine kinase 3 (FLT3) internal tandem duplication (ITD). Patients 18 to 70 years of age with newly diagnosed FLT3-ITD-positive AML were eligible. Primary and key secondary endpoints were event-free survival (EFS) and overall survival (OS). Results were compared with a historical cohort of 415 patients treated on 5 prior AMLSG trials; statistical analysis was performed using a double-robust adjustment with propensity score weighting and covariate adjustment. Results were also compared with patients (18-59 years) treated on the placebo arm of the Cancer and Leukemia Group B (CALGB) 10603/RATIFY trial. The trial accrued 440 patients (18-60 years, n = 312; 61-70 years, n = 128). In multivariate analysis, EFS was significantly in favor of patients treated within the AMLSG 16-10 trial compared with the AMLSG control (hazard ratio [HR], 0.55; P < .001); both in younger (HR, 0.59; P < .001) and older patients (HR, 0.42; P < .001). Multivariate analysis also showed a significant beneficial effect on OS compared with the AMLSG control (HR, 0.57; P < .001) as well as to the CALGB 10603/RATIFY trial (HR, 0.71; P = .005). The treatment effect of midostaurin remained significant in sensitivity analysis including allogeneic HCT as a time-dependent covariate. Addition of midostaurin to chemotherapy was safe in younger and older patients. In comparison with historical controls, the addition of midostaurin to intensive therapy led to a significant improvement in outcome in younger and older patients with AML and FLT3-ITD. This trial is registered at clinicaltrialsregistry.eu as Eudra-CT number 2011-003168-63 and at clinicaltrials.gov as NCT01477606.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures


References
-
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136-1152. - PubMed
-
- Bullinger L, Döhner K, Döhner H. Genomics of acute myeloid leukemia diagnosis and pathways. J Clin Oncol. 2017;35(9):934-946. - PubMed
-
- Nagel G, Weber D, Fromm E, et al. ; German-Austrian AML Study Group (AMLSG) . Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO). Ann Hematol. 2017;96(12): 1993-2003. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

