Temperature or competition: Which has more influence on Mediterranean ant communities?
- PMID: 35486575
- PMCID: PMC9053807
- DOI: 10.1371/journal.pone.0267547
Temperature or competition: Which has more influence on Mediterranean ant communities?
Abstract
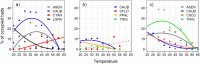
Temperature and competition are two of the main factors determining ant community assemblages. Temperature may allow species to forage more or less efficiently throughout the day (in accordance with the maximum activity temperature of each species). Competition can be observed and quantified from species replacements occurring during resource exploitation. We studied the interspecific competitive interactions of ant communities from the Doñana Biological Reserve (southern Spain). Ants were sampled from pitfall traps and baits in three habitats with contrasted vegetation physiognomy (savin forest, pine forest, and dry scrubland). We measured the temperature during the competitive interactions between species and created a thermal competition index (TCI) to assess the relative contribution of temperature and numerical dominance to the competitive outcomes. Temperature had unequal effects on ant activity in each type of habitat, and modulated competitive interactions. The TCI showed that a species' success during pair interactions (replacements at baits) was driven by the proportion of workers between the two competing species and by the species-specific effect of temperature (how advantageous the temperature change is for each species during bait replacement). During competitive interactions, the effect of temperature (higher values of TCI) and numeric supremacy (higher worker proportion) gave higher success probabilities. Interspecific competitive relationships in these Mediterranean ant communities are habitat dependent and greatly influenced by temperature.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Wilson EO. Success and Dominance in Ecosystems: the Case of Social Insects. In: Kinne O, editor. Excellence in Ecology Vol 2. Oldendorf/Luhe, Germany: Ecology Institute; 1990. p. 104.
-
- Jones CG, Lawton JH, Shachak M. Organisms as ecosystem engineers. Oikos. 1994;69: 373–386.
-
- Davidson DW. Resource discovery versus resource domination in ants: a functional mechanism for breaking the trade-off. Ecol Entomol. 1998;23: 484–490. doi: 10.1046/j.1365-2311.1998.00145.x - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

