Injectable three-dimensional tumor microenvironments to study mechanobiology in ovarian cancer
- PMID: 35487424
- PMCID: PMC10538942
- DOI: 10.1016/j.actbio.2022.04.039
Injectable three-dimensional tumor microenvironments to study mechanobiology in ovarian cancer
Abstract
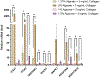
Epithelial ovarian cancers are among the most aggressive forms of gynecological malignancies. Despite the advent of poly adenosine diphosphate-ribose polymerase (PARP) and checkpoint inhibitors, improvement to patient survival has been modest. Limited in part by clinical translation, beneficial therapeutic strategies remain elusive in ovarian cancers. Although elevated levels of extracellular proteins, including collagens, proteoglycans, and glycoproteins, have been linked to chemoresistance, they are often missing from the processes of drug- development and screening. Biophysical and biochemical signaling from the extracellular matrix (ECM) determine cellular phenotype and affect both tumor progression and therapeutic response. However, many state-of-the-art tumor models fail to mimic the complexities of the tumor microenvironment (TME) and omit key signaling components. In this article, two interpenetrating network (IPN) hydrogel scaffold platforms, comprising of alginate-collagen or agarose-collagen, have been characterized for use as 3D in vitro models of epithelial ovarian cancer ECM. These highly tunable, injection mold compatible, and inexpensive IPNs replicate the critical governing physical and chemical signaling present within the ovarian TME. Additionally, an effective and cell-friendly live-cell retrieval method has been established to recover cells post-encapsulation. Lastly, functional mechanotransduction in ovarian cancers was demonstrated by increasing scaffold stiffness within the 3D in vitro ECM models. With these features, the agarose-collagen and alginate-collagen hydrogels provide a robust TME for the study of mechanobiology in epithelial cancers. STATEMENT OF SIGNIFICANCE: Ovarian cancer is the most lethal gynecologic cancer afflicting women today. Here we present the development, characterization, and validation of 3D interpenetrating platforms to shift the paradigm in standard in vitro modeling. These models help elucidate the roles of biophysical and biochemical cues in ovarian cancer progression. The agarose-collagen and alginate-collagen interpenetrating network (IPN) hydrogels are simple to fabricate, inexpensive, and can be modified to create custom mechanical stiffnesses and concentrations of bio-adhesive motifs. Given that investigations into the roles of biophysical characteristics in ovarian cancers have provided incongruent results, we believe that the IPN platforms will be critically important to uncovering molecular drivers. We also expect these platforms to be broadly applicable to studies involving mechanobiology in solid tumors.
Keywords: Agarose; Alginate; Cancers; Collagen; Extracellular matrix; Hydrogel; Injectable; Interpenetrating network; Mechanobiology; Ovarian cancers; Tumor microenvironment.
Copyright © 2022. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


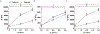


References
-
- Vrabie CD, Petrescu A, Waller M, Dina I, Clinical factors and biomarkers in ovarian tumors development, Rom J Morphol Embryol. 49 (2008) 327–338. - PubMed
-
- Motohara T, Masuda K, Morotti M, Zheng Y, El-Sahhar S, Chong KY, Wietek N, Alsaadi A, Karaminejadranjbar M, Hu Z, Artibani M, Gonzalez LS, Katabuchi H, Saya H, Ahmed AA, An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment, Oncogene. 38 (2019) 2885–2898. 10.1038/s41388-018-0637-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

