Engineering Self-Assembling Protein Nanoparticles for Therapeutic Delivery
- PMID: 35487503
- PMCID: PMC9673152
- DOI: 10.1021/acs.bioconjchem.2c00030
Engineering Self-Assembling Protein Nanoparticles for Therapeutic Delivery
Abstract
Despite remarkable advances over the past several decades, many therapeutic nanomaterials fail to overcome major in vivo delivery barriers. Controlling immunogenicity, optimizing biodistribution, and engineering environmental responsiveness are key outstanding delivery problems for most nanotherapeutics. However, notable exceptions exist including some lipid and polymeric nanoparticles, some virus-based nanoparticles, and nanoparticle vaccines where immunogenicity is desired. Self-assembling protein nanoparticles offer a powerful blend of modularity and precise designability to the field, and have the potential to solve many of the major barriers to delivery. In this review, we provide a brief overview of key designable features of protein nanoparticles and their implications for therapeutic delivery applications. We anticipate that protein nanoparticles will rapidly grow in their prevalence and impact as clinically relevant delivery platforms.
Conflict of interest statement
The authors declare the following competing financial interest(s): N.P.K. is a co-founder, shareholder, paid consultant, and chair of the scientific advisory board of Icosavax, Inc. The King lab has received an unrelated sponsored research agreement from Pfizer.
Figures


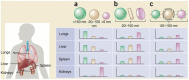

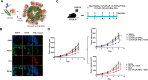

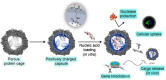

References
-
- Office of the Commissioner. FDA approves first COVID-19 vaccine https://www.fda.gov/news-events/press-announcements/fda-approves-first-c... (accessed 2021 −11 −02).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

