Tenecteplase versus Alteplase for Stroke Thrombolysis Evaluation Trial in the Ambulance (Mobile Stroke Unit-TASTE-A): protocol for a prospective randomised, open-label, blinded endpoint, phase II superiority trial of tenecteplase versus alteplase for ischaemic stroke patients presenting within 4.5 hours of symptom onset to the mobile stroke unit
- PMID: 35487712
- PMCID: PMC9058803
- DOI: 10.1136/bmjopen-2021-056573
Tenecteplase versus Alteplase for Stroke Thrombolysis Evaluation Trial in the Ambulance (Mobile Stroke Unit-TASTE-A): protocol for a prospective randomised, open-label, blinded endpoint, phase II superiority trial of tenecteplase versus alteplase for ischaemic stroke patients presenting within 4.5 hours of symptom onset to the mobile stroke unit
Abstract
Introduction: Mobile stroke units (MSUs) equipped with a CT scanner are increasingly being used to assess and treat stroke patients' prehospital with thrombolysis and transfer them to the most appropriate hospital for ongoing stroke care and thrombectomy when indicated. The effect of MSUs in both reducing the time to reperfusion treatment and improving patient outcomes is now established. There is now an opportunity to improve the efficacy of treatment provided by the MSU. Tenecteplase is a potent plasminogen activator, which may have benefits over the standard of care stroke lytic alteplase. Specifically, in the MSU environment tenecteplase presents practical benefits since it is given as a single bolus and does not require an infusion over an hour like alteplase.
Objective: In this trial, we seek to investigate if tenecteplase, given to patients with acute ischaemic stroke as diagnosed on the MSU, improves the rate of early reperfusion.
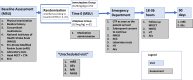
Methods and analysis: TASTE-A is a prospective, randomised, open-label, blinded endpoint (PROBE) phase II trial of patients who had an ischaemic stroke assessed in an MSU within 4.5 hours of symptom onset. The primary endpoint is early reperfusion measured by the post-lysis volume of the CT perfusion lesion performed immediately after hospital arrival.
Ethics and dissemination: The study was approved by the Royal Melbourne Hospital Human Ethics committee. The findings will be published in peer-reviewed journals, presented at academic conferences and disseminated among consumer and healthcare professional audiences.
Trial registration number: NCT04071613.
Keywords: Computed tomography; Neurology; STROKE MEDICINE; Stroke.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Comparison of tenecteplase with alteplase for the early treatment of ischaemic stroke in the Melbourne Mobile Stroke Unit (TASTE-A): a phase 2, randomised, open-label trial.Lancet Neurol. 2022 Jun;21(6):520-527. doi: 10.1016/S1474-4422(22)00171-5. Epub 2022 May 4. Lancet Neurol. 2022. PMID: 35525251 Clinical Trial.
-
Tenecteplase versus alteplase for stroke thrombolysis evaluation (TASTE): A multicentre, prospective, randomized, open-label, blinded-endpoint, controlled phase III non-inferiority trial protocol.Int J Stroke. 2023 Jul;18(6):751-756. doi: 10.1177/17474930231154390. Epub 2023 Feb 2. Int J Stroke. 2023. PMID: 36655938
-
Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open-label, blinded endpoint study.Lancet Neurol. 2015 Apr;14(4):368-76. doi: 10.1016/S1474-4422(15)70017-7. Epub 2015 Feb 26. Lancet Neurol. 2015. PMID: 25726502 Clinical Trial.
-
Tenecteplase Thrombolysis for Acute Ischemic Stroke.Stroke. 2020 Nov;51(11):3440-3451. doi: 10.1161/STROKEAHA.120.029749. Epub 2020 Oct 13. Stroke. 2020. PMID: 33045929 Free PMC article. Review.
-
Tenecteplase for the treatment of acute ischemic stroke: A review of completed and ongoing randomized controlled trials.Int J Stroke. 2018 Dec;13(9):885-892. doi: 10.1177/1747493018790024. Epub 2018 Jul 23. Int J Stroke. 2018. PMID: 30035698 Review.
Cited by
-
Hyperacute ischemic stroke care-Current treatment and future directions.Int J Stroke. 2024 Aug;19(7):718-726. doi: 10.1177/17474930241267353. Int J Stroke. 2024. PMID: 39096172 Free PMC article. Review.
-
Complications of Intravenous Tenecteplase Versus Alteplase for the Treatment of Acute Ischemic Stroke: A Systematic Review and Meta-Analysis.Stroke. 2023 May;54(5):1192-1204. doi: 10.1161/STROKEAHA.122.042335. Epub 2023 Mar 23. Stroke. 2023. PMID: 36951049 Free PMC article.
-
Cost-effectiveness of tenecteplase versus alteplase for stroke thrombolysis evaluation trial in the ambulance.Eur Stroke J. 2023 Jun;8(2):448-455. doi: 10.1177/23969873231165086. Epub 2023 Mar 26. Eur Stroke J. 2023. PMID: 37231684 Free PMC article.
-
Current Status and Future Aspect of Digital Health Innovation in Stroke Prevention and Management.J Atheroscler Thromb. 2025 Jul 1;32(7):775-782. doi: 10.5551/jat.RV22036. Epub 2025 May 9. J Atheroscler Thromb. 2025. PMID: 40350320 Free PMC article. Review.
References
-
- Thomalla G, Sobesky J, Köhrmann M, et al. . Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke; a journal of cerebral circulation 2007;38:313–8. - PubMed
-
- Hacke W, Donnan G, Fieschi C, et al. . Association of outcome with early stroke treatment: pooled analysis of Atlantis, ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768–74. - PubMed
-
- Emberson J, Lees KR, Lyden P, et al. . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. The Lancet 2014;384:1929–35. 10.1016/S0140-6736(14)60584-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical