Achievement rate and predictive factors of the recommended therapeutical target in patients with axial spondyloarthritis who remain on biological therapy: a prospective cohort study in Spain
- PMID: 35487753
- PMCID: PMC9058765
- DOI: 10.1136/bmjopen-2021-057850
Achievement rate and predictive factors of the recommended therapeutical target in patients with axial spondyloarthritis who remain on biological therapy: a prospective cohort study in Spain
Abstract
Objectives: To determine the frequency of sustained remission (R) or low diseas activity (LDA) in patients with axial spondyloarthritis (axSpA) undergoing long-term biological therapy and to analyse predictive factors for achieving these outcomes.
Design: Prospective, observational cohort study.
Setting: Spanish hospital.
Participants: Patients with axSpA who initiated biological treatment between 2003 and 2017.
Intervention: Assessment of demographic and clinical characteristics at the beginning of treatment and disease activity every 6 months up to a maximum of 2 years.
Main outcome measures: Disease activity was measured by Ankylosing Spondylitis Disease Activity Score (ASDAS) and Bath Ankylosing Spondylitis Disease Activity Index and C reactive protein (BASDAI&CRP). Sustained R was defined as ASDAS<1.3 and/or BASDAI <2 and normal CRP while sustained LDA was defined as ASDAS <2.1 and/or BASDAI <4 and normal CRP on at least three consecutive visits.
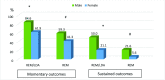
Results: In total 186 patients (66.1% men and 75.3% with radiographic sacroiliitis) were included. Overall, 76.8% of patients achieved ASDAS R/LDA (R53.2%/LDA23.6%) in at least one visit. Forty per cent (R17.6%/LDA22.4%) of the patients fulfilled the sustained ASDAS R/LDA state, whereas only 30.8% maintained this status (R14.8%/LDA15.9%) according to BASDAI&CRP. In the multivariate analysis, male sex (OR=4.01), younger age at the beginning of biological therapy (OR=0.96) and an HLA*B27 positive status (OR=4.30) were associated with achieving sustained ASDAS R/LDA.
Conclusions: In clinical practice, around one-third of patients on biological disease-modifying antirheumatic drugs achieve a sustained R/LDA status, but these rates drop to less than one in five when targeting remission, preventing the use of the latter as a feasible target. Male sex, HLA*B27 positivity and younger age at the beginning of biological therapy are the main predictors for achieving sustained R/LDA.
Keywords: axial spondyloarthritis; bDMARDs; low-disease activity; remission.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DB received grants/speaker/research supports from Roche and Abbvie. CP-R received grants/speaker/research supports from Pfizer, Sanofi, Novartis, Roche and Lilly. RN received grants/speaker/research supports from Novartis, Sanofi Genzyme, Pfizer and Montpellier. IM received grants/research supports from Novartis and speaker’s fees from AbbVie, UCB, Roche and Novartis. DP received grants/research supports from Abbvie, Lilly, MSD and Roche, and had participation in company sponsored speaker’s bureau from Abbvie, Novartis, Lilly, Roche, and MSD. AB received Grant/research support, fees for consultancies or as a speaker for Abbvie, Pfizer, Novartis, BMS, Nordic, Sanofi, Sandoz, Lilly, UCB, Roche. VN-C: consultancy/speaker/research grants from: Abbvie, BMS, Janssen, Lilly, MSD, Novartis, Pfizer, Roche and UCB.
Figures


References
-
- Ward MM, Deodhar A, Gensler LS, et al. Update of the American College of Rheumatology/Spondylitis association of America/Spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and Nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2019;2019. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
