Temperature-sensitive migration dynamics in neutrophil-differentiated HL-60 cells
- PMID: 35488042
- PMCID: PMC9054779
- DOI: 10.1038/s41598-022-10858-w
Temperature-sensitive migration dynamics in neutrophil-differentiated HL-60 cells
Abstract
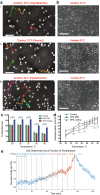
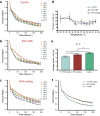
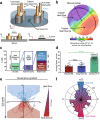
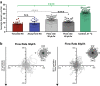
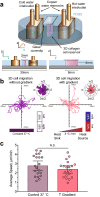
Cell migration plays an essential role in wound healing and inflammatory processes inside the human body. Peripheral blood neutrophils, a type of polymorphonuclear leukocyte (PMN), are the first cells to be activated during inflammation and subsequently migrate toward an injured tissue or infection site. This response is dependent on both biochemical signaling and the extracellular environment, one aspect of which includes increased temperature in the tissues surrounding the inflammation site. In our study, we analyzed temperature-dependent neutrophil migration using differentiated HL-60 cells. The migration speed of differentiated HL-60 cells was found to correlate positively with temperature from 30 to 42 °C, with higher temperatures inducing a concomitant increase in cell detachment. The migration persistence time of differentiated HL-60 cells was higher at lower temperatures (30-33 °C), while the migration persistence length stayed constant throughout the temperature range. Coupled with the increased speed observed at high temperatures, this suggests that neutrophils are primed to migrate more effectively at the elevated temperatures characteristic of inflammation. Temperature gradients exist on both cell and tissue scales. Taking this into consideration, we also investigated the ability of differentiated HL-60 cells to sense and react to the presence of temperature gradients, a process known as thermotaxis. Using a two-dimensional temperature gradient chamber with a range of 27-43 °C, we observed a migration bias parallel to the gradient, resulting in both positive and negative thermotaxis. To better mimic the extracellular matrix (ECM) environment in vivo, a three-dimensional collagen temperature gradient chamber was constructed, allowing observation of biased neutrophil-like differentiated HL-60 migration toward the heat source.
© 2022. The Author(s).
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

