Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy
- PMID: 35488343
- PMCID: PMC9052603
- DOI: 10.1186/s12951-022-01421-w
Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy
Abstract
Background: Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) characterized by diffuse inflammation of the colonic mucosa and a relapsing and remitting course. The current therapeutics are only modestly effective and carry risks for unacceptable adverse events, and thus more effective approaches to treat UC is clinically needed.
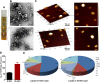
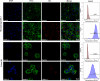
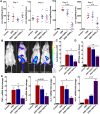
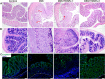
Results: For this purpose, turmeric-derived nanoparticles with a specific population (TDNPs 2) were characterized, and their targeting ability and therapeutic effects against colitis were investigated systematically. The hydrodynamic size of TDNPs 2 was around 178 nm, and the zeta potential was negative (- 21.7 mV). Mass spectrometry identified TDNPs 2 containing high levels of lipids and proteins. Notably, curcumin, the bioactive constituent of turmeric, was evidenced in TDNPs 2. In lipopolysaccharide (LPS)-induced acute inflammation, TDNPs 2 showed excellent anti-inflammatory and antioxidant properties. In mice colitis models, we demonstrated that orally administrated of TDNPs 2 could ameliorate mice colitis and accelerate colitis resolution via regulating the expression of the pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1β, and antioxidant gene, HO-1. Results obtained from transgenic mice with NF-κB-RE-Luc indicated that TDNPs 2-mediated inactivation of the NF-κB pathway might partially contribute to the protective effect of these particles against colitis.
Conclusion: Our results suggest that TDNPs 2 from edible turmeric represent a novel, natural colon-targeting therapeutics that may prevent colitis and promote wound repair in colitis while outperforming artificial nanoparticles in terms of low toxicity and ease of large-scale production.
Keywords: NF-κB pathway; Nanotherapeutics; Oral administration; Turmeric-derived nanoparticles; Ulcerative colitis.
© 2022. The Author(s).
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures





References
-
- Shivaji UN, Nardone OM, Cannatelli R, Smith SC, Ghosh S, Iacucci M. Small molecule oral targeted therapies in ulcerative colitis. Lancet Gastroenterol. 2020;5:850–861. - PubMed
-
- Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74. - PubMed
-
- Chung CH, Jung W, Keum H, Kim TW, Jon S. Nanoparticles derived from the natural antioxidant rosmarinic acid ameliorate acute inflammatory bowel disease. ACS Nano. 2020;14:6887–6896. - PubMed
-
- Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

