Increased mTORC2 pathway activation in lymph nodes of iMCD-TAFRO
- PMID: 35488725
- PMCID: PMC9170805
- DOI: 10.1111/jcmm.17251
Increased mTORC2 pathway activation in lymph nodes of iMCD-TAFRO
Abstract
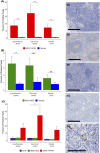
Idiopathic multicentric Castleman disease (iMCD) is a rare and life-threatening haematologic disorder involving polyclonal lymphoproliferation and organ dysfunction due to excessive cytokine production, including interleukin-6 (IL-6). Clinical trial and real-world data demonstrate that IL-6 inhibition is effective in 34-50% of patients. mTOR, which functions through mTORC1 and mTORC2, is a recently discovered therapeutic target. The mTOR inhibitor sirolimus, which preferentially inhibits mTORC1, has led to sustained remission in a small cohort of anti-IL-6-refractory iMCD patients with thrombocytopenia, anasarca, fever, renal dysfunction and organomegaly (iMCD-TAFRO). However, sirolimus has not shown uniform effect, potentially due to its limited mTORC2 inhibition. To investigate mTORC2 activation in iMCD, we quantified the mTORC2 effector protein pNDRG1 by immunohistochemistry of lymph node tissue from six iMCD-TAFRO and eight iMCD patients who do not meet TAFRO criteria (iMCD-not-otherwise-specified; iMCD-NOS). mTORC2 activation was increased in all regions of iMCD-TAFRO lymph nodes and the interfollicular space of iMCD-NOS compared with control tissue. Immunohistochemistry also revealed increased pNDRG1 expression in iMCD-TAFRO germinal centres compared with autoimmune lymphoproliferative syndrome (ALPS), an mTOR-driven, sirolimus-responsive lymphoproliferative disorder, and comparable staining between iMCD-NOS and ALPS. These results suggest increased mTORC2 activity in iMCD and that dual mTORC1/mTORC2 inhibitors may be a rational therapeutic approach.
Keywords: Castleman disease; TAFRO; autoimmune lymphoproliferative syndrome; iMCD; idiopathic multicentric Castleman disease; mTOR; mTORC2; pNDRG1.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
D.C.F. has received research funding for the ACCELERATE registry (NCT02817997) from EUSA Pharma and consulting fees from EUSA Pharma, and Pfizer provides study drug with no associated research funding for the clinical trial of sirolimus (NCT03933904). D.C.F. has two provisional patents pending related to the diagnosis and treatment of iMCD. The remaining authors declare no competing interests.
Figures
References
-
- Iwaki N, Fajgenbaum DC, Nabel CS, et al. Clinicopathological analysis of TAFRO syndrome demonstrates a distinct subtype of HHV‐8‐negative multicentric Castleman disease. Am J Hematol. 2015;91(2):220‐226. - PubMed
-
- Dispenzieri A, Fajgenbaum DC. Overview of Castleman disease. Blood. 2020;135(16):1353‐1364. - PubMed
-
- Van Rhee F, Wong RS, Munshi N, et al. Siltuximab for multicentric Castleman’s disease: a randomized, double‐blind, placebo‐controlled trial. Lancet Oncol. 2014;15(9):966‐974. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

