Unique seeding profiles and prion-like propagation of synucleinopathies are highly dependent on the host in human α-synuclein transgenic mice
- PMID: 35488930
- PMCID: PMC9199436
- DOI: 10.1007/s00401-022-02425-4
Unique seeding profiles and prion-like propagation of synucleinopathies are highly dependent on the host in human α-synuclein transgenic mice
Abstract
α-synuclein (αSyn) is an intrinsically disordered protein which can undergo structural transformations, resulting in the formation of stable, insoluble fibrils. αSyn amyloid-type nucleation can be induced by misfolded 'seeds' serving as a conformational template, tantamount to the prion-like mechanism. Accumulation of αSyn inclusions is a key feature of dementia with Lewy bodies (DLB) and multiple system atrophy (MSA), and are found as additional pathology in Alzheimer's disease (AD) such as AD with amygdala predominant Lewy bodies (AD/ALB). While these disorders accumulate the same pathological protein, they exhibit heterogeneity in clinical and histological features; however, the mechanism(s) underlying this variability remains elusive. Accruing data from human autopsy studies, animal inoculation modeling, and in vitro characterization experiments, have lent credence to the hypothesis that conformational polymorphism of the αSyn amyloid-type fibril structure results in distinct "strains" with categorical infectivity traits. Herein, we directly compare the seeding abilities and outcome of human brain lysates from these diseases, as well as recombinant preformed human αSyn fibrils by the intracerebral inoculation of transgenic mice overexpressing either human wild-type αSyn or human αSyn with the familial A53T mutation. Our study has revealed that the initiating inoculum heavily dictates the phenotypic and pathological course of disease. Interestingly, we have also established relevant host-dependent distinctions between propagation profiles, including burden and spread of inclusion pathology throughout the neuroaxis, as well as severity of neurological symptoms. These findings provide compelling evidence supporting the hypothesis that diverse prion-type conformers may explain the variability seen in synucleinopathies.
Keywords: Alzheimer’s disease with amygdala predominant Lewy bodies; Amyloid; Dementia with Lewy bodies; Multiple system atrophy; Prion; Strains; Synucleinopathy; α-synuclein.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Figures

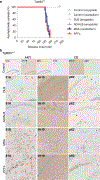




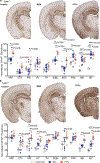



References
-
- Aguzzi A, Rajendran L (2009) The Transcellular Spread of Cytosolic Amyloids, Prions, and Prionoids. Neuron 64:783–790 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

