Production of flavor compounds from rice bran by yeasts metabolisms of Kluyveromyces marxianus and Debaryomyces hansenii
- PMID: 35488980
- PMCID: PMC9433634
- DOI: 10.1007/s42770-022-00766-6
Production of flavor compounds from rice bran by yeasts metabolisms of Kluyveromyces marxianus and Debaryomyces hansenii
Abstract
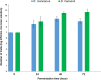
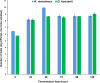
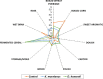
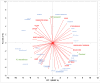
The aim of this study was to evaluate the biosynthesis of flavor compounds from rice bran by fermentation facilitated by Kluyveromyces marxianus and Debaryomyces hansenii. The growth of both yeasts was assessed by specific growth rates and doubling time. The biosynthesis of flavor compounds was evaluated by gas chromatography-olfactometry (GC-O), gas chromatography-mass spectrometry (GC-MS), and Spectrum™ sensory analysis. The specific growth rate (µ) and doubling time (td) of K. marxianus was calculated as 0.16/h and 4.21h, respectively, whereas that of D. hansenii was determined as 0.13/h and 5.33h, respectively. K. marxianus and D. hansenii produced significant levels of higher alcohols and acetate esters from rice bran. Results showed that K. marxianus can produce 827.27 µg/kg of isoamyl alcohol, 169.77 µg/kg of phenyl ethyl alcohol, and 216.08 µg/kg of phenyl ethyl acetate after 24-h batch fermentation. A significant amount of isovaleric acid was also synthesized by K. marxianus (4013 µg/kg) after the batch fermentation of 96 h. 415.64 µg/kg of isoamyl alcohol and 135.77 µg/kg of phenyl ethyl acetate was determined in rice bran fermented by D. hansenii after 24-h fermentation. Fermented cereals and rose were the characteristic flavor descriptors of the fermented rice bran samples. Rose flavor in fermented rice bran samples was found to be associated with phenyl ethyl alcohol, phenyl ethyl acetate, isoamyl acetate, and guaiacol. Thus, the findings of this study demonstrate that the valorization of rice bran can be achieved with the production of natural flavor compounds by yeast metabolism.
Keywords: Bioflavor; Gas chromatography–olfactometry; Microbial fermentation; Sensory analysis; Yeast metabolism.
© 2022. The Author(s) under exclusive licence to Sociedade Brasileira de Microbiologia.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Hosoglu MI, Guneser O, Yuceer YK (2018) Different bioengineering approaches on production of bioflavor compounds. In: Grumezescu AM, Holban AM (eds) The role of materials science in food bioengineering Volume 19, 1st edn. Academic Press, Cambridge, pp 37–71. 10.1016/B978-0-12-811448-3.00002-4
-
- Scragg AH (2007) The production of flavours by plant cell cultures. In: Berger RG (ed) Flavours and fragrances: chemistry, bioprocessing and sustainability, 1st edn. Springer, Berlin-Heidelberg, pp 599–614. 10.1007/978-3-540-49339-6_25
-
- Ziegler H (2007) Introduction. In: Ziegler H (ed) Flavourings: production, composition, applications regulations, 2nd edn. Wiley-VCH Verlag GmbH&Co. KGaA, Weinheim, pp 1–13. 10.1002/9783527611454.ch1
-
- Bettio G, Zardo LC, Rosa CA, Záchia Ayub MA (2021) Bioconversion of ferulic acid into aroma compounds by newly isolated yeast strains of the Latin American biodiversity. Biotechnol Prog 37 e3067. 10.1002/btpr.3067 - PubMed
-
- Ben Akacha N, Gargouri M. Microbial and enzymatic technologies used for the production of natural aroma compounds: synthesis, recovery modeling, and bioprocesses. Food Bioprod Process. 2015;94:675–706. doi: 10.1016/j.fbp.2014.09.011. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

