DNA binding by polycomb-group proteins: searching for the link to CpG islands
- PMID: 35489059
- PMCID: PMC9122586
- DOI: 10.1093/nar/gkac290
DNA binding by polycomb-group proteins: searching for the link to CpG islands
Abstract
Polycomb group proteins predominantly exist in polycomb repressive complexes (PRCs) that cooperate to maintain the repressed state of thousands of cell-type-specific genes. Targeting PRCs to the correct sites in chromatin is essential for their function. However, the mechanisms by which PRCs are recruited to their target genes in mammals are multifactorial and complex. Here we review DNA binding by polycomb group proteins. There is strong evidence that the DNA-binding subunits of PRCs and their DNA-binding activities are required for chromatin binding and CpG targeting in cells. In vitro, CpG-specific binding was observed for truncated proteins externally to the context of their PRCs. Yet, the mere DNA sequence cannot fully explain the subset of CpG islands that are targeted by PRCs in any given cell type. At this time we find very little structural and biophysical evidence to support a model where sequence-specific DNA-binding activity is required or sufficient for the targeting of CpG-dinucleotide sequences by polycomb group proteins while they are within the context of their respective PRCs, either PRC1 or PRC2. We discuss the current knowledge and open questions on how the DNA-binding activities of polycomb group proteins facilitate the targeting of PRCs to chromatin.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
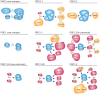

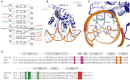
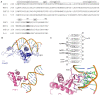


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

