Cannabinoid receptor 1 antagonist genistein attenuates marijuana-induced vascular inflammation
- PMID: 35489334
- PMCID: PMC9400797
- DOI: 10.1016/j.cell.2022.04.005
Cannabinoid receptor 1 antagonist genistein attenuates marijuana-induced vascular inflammation
Erratum in
-
Cannabinoid receptor 1 antagonist genistein attenuates marijuana-induced vascular inflammation.Cell. 2022 Jun 23;185(13):2387-2389. doi: 10.1016/j.cell.2022.06.006. Cell. 2022. PMID: 35750035 No abstract available.
Abstract
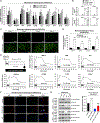
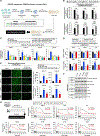
Epidemiological studies reveal that marijuana increases the risk of cardiovascular disease (CVD); however, little is known about the mechanism. Δ9-tetrahydrocannabinol (Δ9-THC), the psychoactive component of marijuana, binds to cannabinoid receptor 1 (CB1/CNR1) in the vasculature and is implicated in CVD. A UK Biobank analysis found that cannabis was an risk factor for CVD. We found that marijuana smoking activated inflammatory cytokines implicated in CVD. In silico virtual screening identified genistein, a soybean isoflavone, as a putative CB1 antagonist. Human-induced pluripotent stem cell-derived endothelial cells were used to model Δ9-THC-induced inflammation and oxidative stress via NF-κB signaling. Knockdown of the CB1 receptor with siRNA, CRISPR interference, and genistein attenuated the effects of Δ9-THC. In mice, genistein blocked Δ9-THC-induced endothelial dysfunction in wire myograph, reduced atherosclerotic plaque, and had minimal penetration of the central nervous system. Genistein is a CB1 antagonist that attenuates Δ9-THC-induced atherosclerosis.
Keywords: Billy Martin tetrad; G protein-coupled receptor; GPCR; UK Biobank; atherosclerosis; cardiovascular disease; human-induced pluripotent stem cell; in silico drug screening; in vivo ligand binding; marijuana.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.C.W. is a co-founder and SAB of Greenstone Biosciences, but the work was done independently. B.K.K. is a co-founder and SAB of ConfometRx, but the work was done independently.
Figures





Comment in
-
Getting to the heart of cannabis health risks.Cell. 2022 May 12;185(10):1623-1625. doi: 10.1016/j.cell.2022.04.018. Cell. 2022. PMID: 35561662
-
CNR1 antagonism attenuates cannabis-induced atherosclerosis.Nat Rev Cardiol. 2022 Jul;19(7):432. doi: 10.1038/s41569-022-00724-7. Nat Rev Cardiol. 2022. PMID: 35581336 No abstract available.
-
Marijuana and endothelial dysfunction: new mechanism and therapy.Trends Mol Med. 2022 Aug;28(8):613-615. doi: 10.1016/j.molmed.2022.05.009. Epub 2022 Jun 11. Trends Mol Med. 2022. PMID: 35701315
-
The cannabis paradox: contrasting role for marijuana in cardiovascular disease.Signal Transduct Target Ther. 2022 Sep 5;7(1):309. doi: 10.1038/s41392-022-01160-2. Signal Transduct Target Ther. 2022. PMID: 36064830 Free PMC article. No abstract available.
References
-
- Akira S, Uematsu S, and Takeuchi O (2006). Pathogen recognition and innate immunity. Cell 124, 783–801. - PubMed
-
- Ardigo D, Assimes TL, Fortmann SP, Go AS, Hlatky M, Hytopoulos E, Iribarren C, Tsao PS, Tabibiazar R, and Quertermous T; ADVANCE Investigators (2007). Circulating chemokines accurately identify individuals with clinically significant atherosclerotic heart disease. Physiol. Genomics 31, 402–409. - PubMed
-
- Baba O, Huang LH, Elvington A, Szpakowska M, Sultan D, Heo GS, Zhang X, Luehmann H, Detering L, Chevigne A, et al. (2021). CXCR4-binding positron emission tomography tracers link monocyte recruitment and endothelial injury in murine atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 41, 822–836. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

