Laser ablation: Heating up the anti-tumor response in the intracranial compartment
- PMID: 35489652
- PMCID: PMC10589123
- DOI: 10.1016/j.addr.2022.114311
Laser ablation: Heating up the anti-tumor response in the intracranial compartment
Abstract
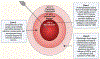
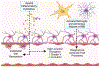
Immunotherapies, such as immune checkpoint inhibition (ICI), have had limited success in treating intracranial malignancies. These failures are due partly to the restrictive blood-brain-barrier (BBB), the profound tumor-dependent induction of local and systemic immunosuppression, and immune evasion exhibited by these tumors. Therefore, novel approaches must be explored that aim to overcome these stringent barriers. LITT is an emerging treatment for brain tumors that utilizes thermal ablation to kill tumor cells. LITT provides an additional therapeutic benefit by synergizing with ICI and systemic chemotherapies to strengthen the anti-tumor immune response. This synergistic relationship involves transient disruption of the BBB and local augmentation of immune function, culminating in increased CNS drug penetrance and improved anti-tumor immunity. In this review, we will provide an overview of the challenges facing immunotherapy for brain tumors, and discuss how LITT may synergize with the endogenous anti-tumor response to improve the efficacy of ICI.
Keywords: Anti-tumor immunity; Blood brain barrier; Brain metastases; Checkpoint blockade; Checkpoint inhibition; Glioblastoma; Glioma; Hyperthermia; Immunotherapy; Intracranial tumors; LITT laser interstitial thermal therapy.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Schreiber RD, Old LJ, and Smyth MJ, Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science, 2011. 331(6024): p. 1565–1570. - PubMed
-
- Dunn GP, et al., Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunology, 2002. 3(11): p. 991–998. - PubMed
-
- Dunn GP, Fecci PE, and Curry WT, Cancer immunoediting in malignant glioma. Neurosurgery, 2012. 71(2): p. 201–22; discussion 222-3. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

