Virtual Ontogeny of Cortical Growth Preceding Mental Illness
- PMID: 35489875
- PMCID: PMC11080987
- DOI: 10.1016/j.biopsych.2022.02.959
Virtual Ontogeny of Cortical Growth Preceding Mental Illness
Abstract
Background: Morphology of the human cerebral cortex differs across psychiatric disorders, with neurobiology and developmental origins mostly undetermined. Deviations in the tangential growth of the cerebral cortex during pre/perinatal periods may be reflected in individual variations in cortical surface area later in life.
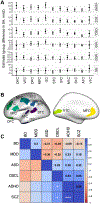
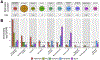
Methods: Interregional profiles of group differences in surface area between cases and controls were generated using T1-weighted magnetic resonance imaging from 27,359 individuals including those with attention-deficit/hyperactivity disorder, autism spectrum disorder, bipolar disorder, major depressive disorder, schizophrenia, and high general psychopathology (through the Child Behavior Checklist). Similarity of interregional profiles of group differences in surface area and prenatal cell-specific gene expression was assessed.
Results: Across the 11 cortical regions, group differences in cortical area for attention-deficit/hyperactivity disorder, schizophrenia, and Child Behavior Checklist were dominant in multimodal association cortices. The same interregional profiles were also associated with interregional profiles of (prenatal) gene expression specific to proliferative cells, namely radial glia and intermediate progenitor cells (greater expression, larger difference), as well as differentiated cells, namely excitatory neurons and endothelial and mural cells (greater expression, smaller difference). Finally, these cell types were implicated in known pre/perinatal risk factors for psychosis. Genes coexpressed with radial glia were enriched with genes implicated in congenital abnormalities, birth weight, hypoxia, and starvation. Genes coexpressed with endothelial and mural genes were enriched with genes associated with maternal hypertension and preterm birth.
Conclusions: Our findings support a neurodevelopmental model of vulnerability to mental illness whereby prenatal risk factors acting through cell-specific processes lead to deviations from typical brain development during pregnancy.
Keywords: Cortical growth; Cortical surface area; Mental illness; Neurodevelopment; Neurogenesis; Psychiatric disorders.
Copyright © 2022 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, et al. (2009): Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513:532–541. - PubMed
-
- Rakic P (1988): Specification of cerebral cortical areas. Science 241:170–176. - PubMed
Publication types
MeSH terms
Grants and funding
- P50 MH094268/MH/NIMH NIH HHS/United States
- U01 DA041120/DA/NIDA NIH HHS/United States
- U01 DA041093/DA/NIDA NIH HHS/United States
- R01 MH083968/MH/NIMH NIH HHS/United States
- RF1 NS114628/NS/NINDS NIH HHS/United States
- U01 DA051038/DA/NIDA NIH HHS/United States
- T32 AG058507/AG/NIA NIH HHS/United States
- U01 DA051016/DA/NIDA NIH HHS/United States
- R01 MH107730/MH/NIMH NIH HHS/United States
- U01 DA041106/DA/NIDA NIH HHS/United States
- S10 OD023696/OD/NIH HHS/United States
- U24 DA041147/DA/NIDA NIH HHS/United States
- R37 MH101495/MH/NIMH NIH HHS/United States
- R01 NS114628/NS/NINDS NIH HHS/United States
- U01 DA051039/DA/NIDA NIH HHS/United States
- HHSN271200800009C/DA/NIDA NIH HHS/United States
- R01 MH116948/MH/NIMH NIH HHS/United States
- P41 RR008079/RR/NCRR NIH HHS/United States
- U01 DA051018/DA/NIDA NIH HHS/United States
- R01 EB015611/EB/NIBIB NIH HHS/United States
- UL1 TR001872/TR/NCATS NIH HHS/United States
- U24 DA041123/DA/NIDA NIH HHS/United States
- R01 MH105660/MH/NIMH NIH HHS/United States
- U01 DA041134/DA/NIDA NIH HHS/United States
- R01 MH116147/MH/NIMH NIH HHS/United States
- U01 DA041022/DA/NIDA NIH HHS/United States
- U01 DA041156/DA/NIDA NIH HHS/United States
- U01 DA050987/DA/NIDA NIH HHS/United States
- R01 MH117601/MH/NIMH NIH HHS/United States
- U01 DA051037/DA/NIDA NIH HHS/United States
- U01 MH108148/MH/NIMH NIH HHS/United States
- U54 EB020403/EB/NIBIB NIH HHS/United States
- U01 DA041025/DA/NIDA NIH HHS/United States
- U01 DA050989/DA/NIDA NIH HHS/United States
- U01 DA041089/DA/NIDA NIH HHS/United States
- RF1 MH123163/MH/NIMH NIH HHS/United States
- U01 DA050988/DA/NIDA NIH HHS/United States
- U24 RR021992/RR/NCRR NIH HHS/United States
- U01 DA041117/DA/NIDA NIH HHS/United States
- U01 DA041028/DA/NIDA NIH HHS/United States
- U01 DA041048/DA/NIDA NIH HHS/United States
- R01 AA012207/AA/NIAAA NIH HHS/United States
- K23 MH090421/MH/NIMH NIH HHS/United States
- U01 DA041148/DA/NIDA NIH HHS/United States
- R01 MH121246/MH/NIMH NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R61 AT009864/AT/NCCIH NIH HHS/United States
- MC_PC_17209/MRC_/Medical Research Council/United Kingdom
- U24 RR025736/RR/NCRR NIH HHS/United States
- R01 MH085734/MH/NIMH NIH HHS/United States
- U01 DA041174/DA/NIDA NIH HHS/United States
- R21 AT009173/AT/NCCIH NIH HHS/United States

