Group B streptococcus infection during pregnancy and infancy: estimates of regional and global burden
- PMID: 35490693
- PMCID: PMC9090904
- DOI: 10.1016/S2214-109X(22)00093-6
Group B streptococcus infection during pregnancy and infancy: estimates of regional and global burden
Erratum in
-
Correction to Lancet Glob Health 2022; 10: e807-19.Lancet Glob Health. 2022 Jul;10(7):e960. doi: 10.1016/S2214-109X(22)00235-2. Epub 2022 May 12. Lancet Glob Health. 2022. PMID: 35569488 Free PMC article. No abstract available.
Abstract
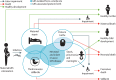
Background: Group B streptococcus (GBS) colonisation during pregnancy can lead to invasive GBS disease (iGBS) in infants, including meningitis or sepsis, with a high mortality risk. Other outcomes include stillbirths, maternal infections, and prematurity. There are data gaps, notably regarding neurodevelopmental impairment (NDI), especially after iGBS sepsis, which have limited previous global estimates. In this study, we aimed to address this gap using newly available multicountry datasets.
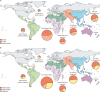
Methods: We collated and meta-analysed summary data, primarily identified in a series of systematic reviews published in 2017 but also from recent studies on NDI and stillbirths, using Bayesian hierarchical models, and estimated the burden for 183 countries in 2020 regarding: maternal GBS colonisation, iGBS cases and deaths in infants younger than 3 months, children surviving iGBS affected by NDI, and maternal iGBS cases. We analysed the proportion of stillbirths with GBS and applied this to the UN-estimated stillbirth risk per country. Excess preterm births associated with maternal GBS colonisation were calculated using meta-analysis and national preterm birth rates.
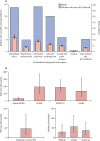
Findings: Data from the seven systematic reviews, published in 2017, that informed the previous burden estimation (a total of 515 data points) were combined with new data (17 data points) from large multicountry studies on neurodevelopmental impairment (two studies) and stillbirths (one study). A posterior median of 19·7 million (95% posterior interval 17·9-21·9) pregnant women were estimated to have rectovaginal colonisation with GBS in 2020. 231 800 (114 100-455 000) early-onset and 162 200 (70 200-394 400) late-onset infant iGBS cases were estimated to have occurred. In an analysis assuming a higher case fatality rate in the absence of a skilled birth attendant, 91 900 (44 800-187 800) iGBS infant deaths were estimated; in an analysis without this assumption, 58 300 (26 500-125 800) infant deaths from iGBS were estimated. 37 100 children who recovered from iGBS (14 600-96 200) were predicted to develop moderate or severe NDI. 40 500 (21 500-66 200) maternal iGBS cases and 46 200 (20 300-111 300) GBS stillbirths were predicted in 2020. GBS colonisation was also estimated to be potentially associated with considerable numbers of preterm births.
Interpretation: Our analysis provides a comprehensive assessment of the pregnancy-related GBS burden. The Bayesian approach enabled coherent propagation of uncertainty, which is considerable, notably regarding GBS-associated preterm births. Our findings on both the acute and long-term consequences of iGBS have public health implications for understanding the value of investment in maternal GBS immunisation and other preventive strategies.
Funding: Bill & Melinda Gates Foundation.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The Department of Clinical Epidemiology of Aarhus University receives funding from private and public institutions in the form of institutional research grants to (and administered by) Aarhus University; none of these grants has any relation to the present study. SAM declares funding from Astrazeneca, the Bill & Melinda Gates Foundation, GlaxoSmithKline, Minervax, Novavax, Pfizer, and the South Africa Medical Research Council; in particular, SAM delares funding to his institution from Pfizer for epidemiology studies on group B streptococcus (GBS) and a clinical trial on the GBS vaccine, and from the Bill & Melinda Gates Foundation on GBS epidemiology. FS declares employment by the UK National Screening Committee, which developed the policy recommendation for maternal GBS screening. CT declares a consulting fee from WHO for drafting a report on the Full Value of Vaccine Assessment for GBS vaccines, which is related to the current manuscript. RL declares participation on an advisory board for Janssen and Pfizer; payment for lectures from Reckitt; and grants to Fundación INFANT from the Bill & Melinda Gates Foundation and PATH. All other authors declare no competing interests.
Figures
Comment in
-
Variation of invasive neonatal GBS disease across the regions.Lancet Glob Health. 2022 Jun;10(6):e776-e777. doi: 10.1016/S2214-109X(22)00182-6. Epub 2022 Apr 28. Lancet Glob Health. 2022. PMID: 35490694 No abstract available.
-
The urgent need to recognize and properly address prenatal-onset group B Streptococcus disease.Int J Infect Dis. 2022 Nov;124:168-170. doi: 10.1016/j.ijid.2022.10.016. Epub 2022 Oct 13. Int J Infect Dis. 2022. PMID: 36243281 No abstract available.
References
-
- Melin P, Efstratiou A. Group B streptococcal epidemiology and vaccine needs in developed countries. Vaccine. 2013;31(suppl 4):D31–D42. - PubMed
-
- Franciosi RA, Knostman JD, Zimmerman RA. Group B streptococcal neonatal and infant infections. J Pediatr. 1973;82:707–718. - PubMed
-
- Eickhoff TC, Klein JO, Daly AK, Ingall D, Finland M. Neonatal sepsis and other infections due to group B beta-hemolytic streptococci. N Engl J Med. 1964;271:1221–1228. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

